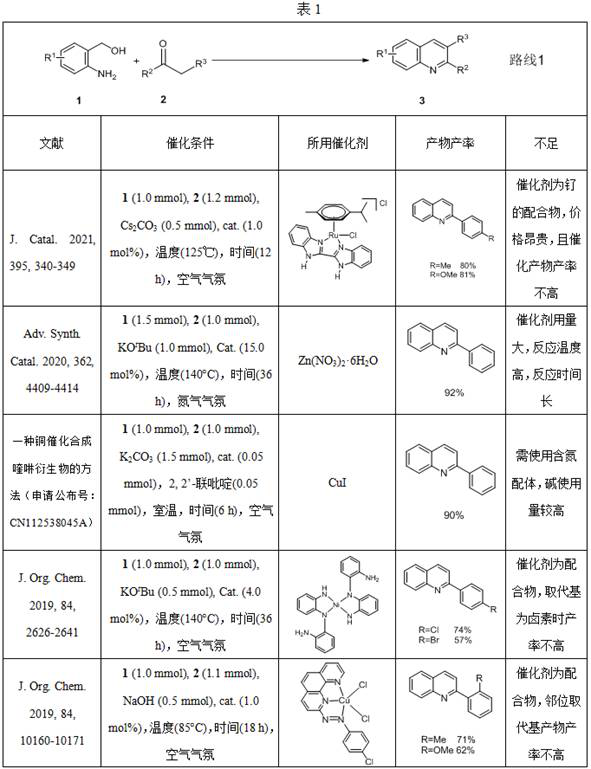
Method for synthesizing quinoline and quinazoline compounds through cobalt catalysis
A technology of quinazolines and compounds, which is applied in the field of organic synthesis, can solve problems such as limiting industrial application prospects, and achieve the effects of low cost, high catalytic activity, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
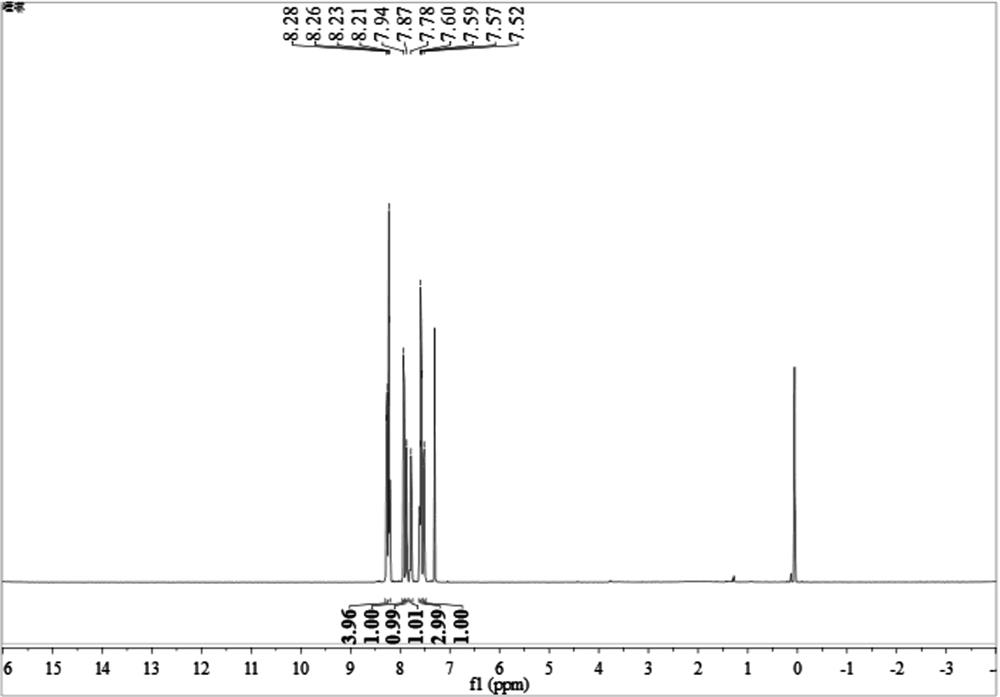
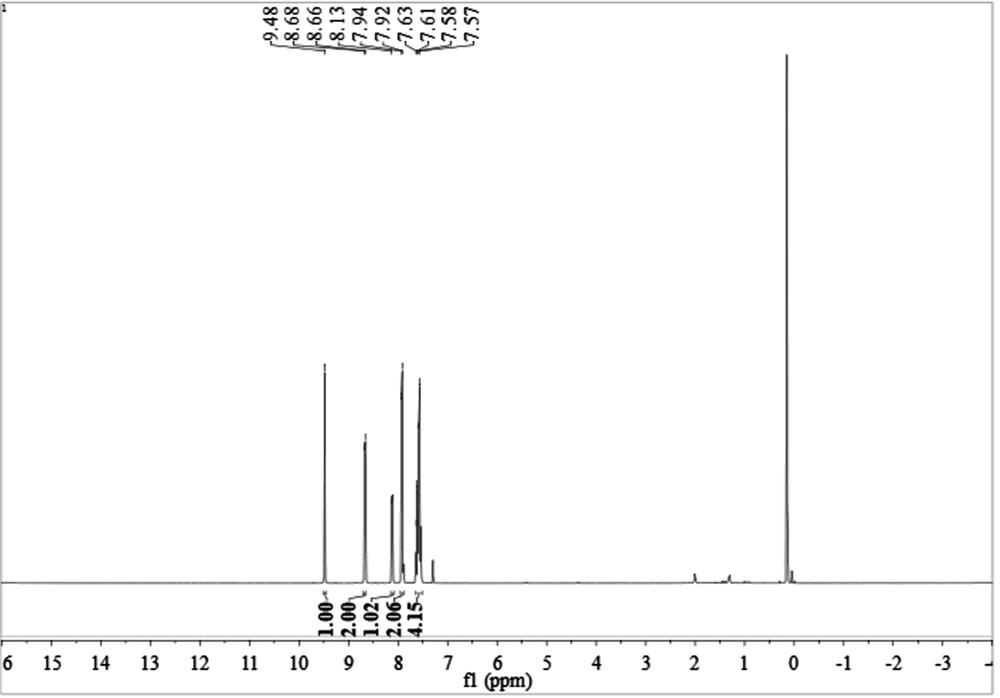
[0049] 2-aminobenzyl alcohol 1a (123.2 mg, 1.0 mmol), acetophenone 2a (120.2 mg, 1.0 mmol), Co(OAc) were added to the reaction vessel 2 4H 2 O (19.9 mg, 0.08 mmol) and KO t Bu (112.0 mg, 1.0 mmol). Under an argon atmosphere, 2 mL of toluene was added and heated at 110 °C for 12 h. After cooling to room temperature, 10 mL of water was added, and the mixture was extracted with EtOAc (3×10 mL). The combined organic phases were concentrated under reduced pressure. The residue was then purified by flash column chromatography (petroleum ether:ethyl acetate 100:1, v / v) to give 3a (198.9 mg, yield 97%) as a white solid, whose NMR spectrum was shown in figure 1 shown.
Embodiment 2
[0051] 2-aminobenzyl alcohol 1a (123.2 mg, 1.0 mmol), acetophenone 2a (120.2 mg, 1.0 mmol), Co(OAc) were added to the reaction vessel 2 • 4H 2 O (19.9 mg, 0.08 mmol) and KO t Bu (78.4 mg, 0.7 mmol). Under an argon atmosphere, 2 mL of toluene was added and heated at 110 °C for 12 h. After cooling to room temperature, 10 mL of water was added, and the mixture was extracted with EtOAc (3×10 mL). The combined organic phases were concentrated under reduced pressure. The residue was then purified by flash column chromatography (petroleum ether:ethyl acetate 100:1, v / v) to obtain white solid 3a (168.2 mg, yield 82%).
Embodiment 3
[0053] 2-aminobenzyl alcohol 1a (123.2 mg, 1.0 mmol), acetophenone 2a (144.2 mg, 1.2 mmol), Co(OAc) were added to the reaction vessel 2 • 4H 2 O (24.9 mg, 0.1 mmol) and KO t Bu (112.0 mg, 1.0 mmol). Under an argon atmosphere, 2 mL of toluene was added and heated at 110 °C for 12 h. After cooling to room temperature, 10 mL of water was added, and the mixture was extracted with EtOAc (3×10 mL). The combined organic phases were concentrated under reduced pressure. The residue was then purified by flash column chromatography (petroleum ether:ethyl acetate 100:1, v / v) to obtain white solid 3a (196.9 mg, yield 96%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com