Primer-probe combination and detection kit for detecting new coronavirus and pneumonia pathogenic bacteria
A technology for detection kits and primer probes, applied in the field of primer probe combinations and detection kits for the detection of novel coronavirus and pneumonia pathogenic bacteria, to achieve high sensitivity, good sensitivity and specificity, efficient screening and triage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1 Preparation of detection kits for novel coronavirus and pneumonia pathogens
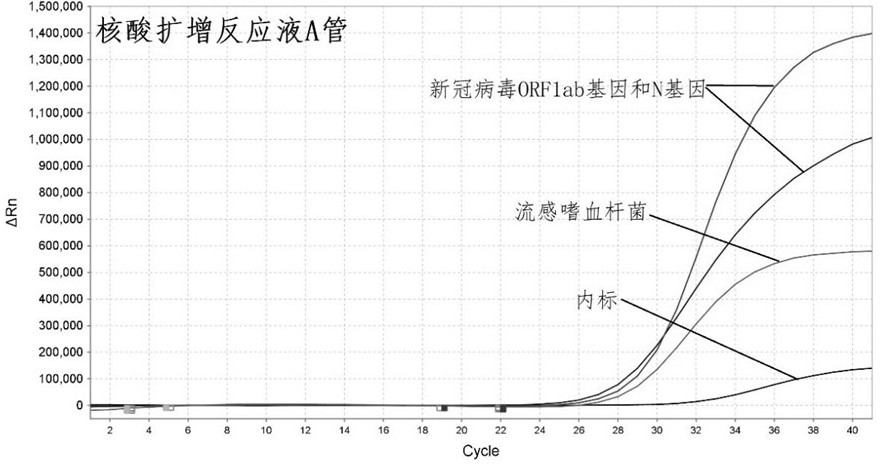
[0089] Prepare nucleic acid amplification reaction solution according to Table 2 and Table 3 below.
[0090] Table 2: Components and concentrations of nucleic acid amplification reaction solution A
[0091]
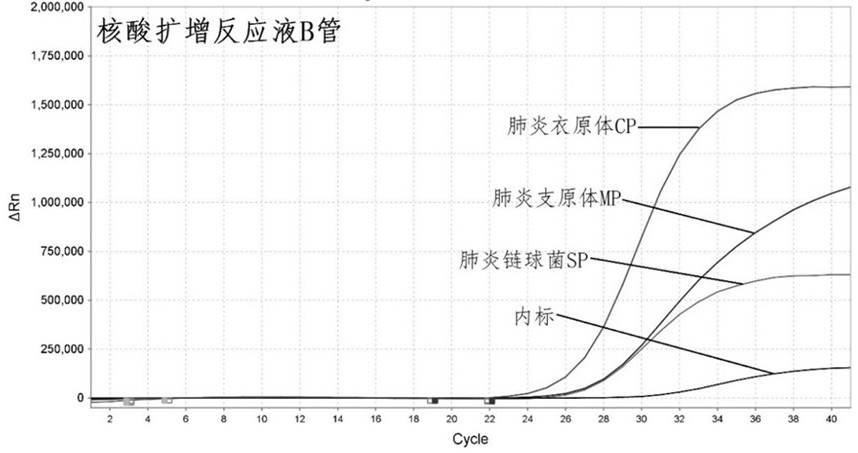
[0092] Table 3 Component B and concentration of nucleic acid amplification reaction solution
[0093]
[0094] Prepare the enzyme mixture according to Table 4.
[0095] Table 4
[0096]
[0097] Positive controls were prepared according to Table 5.
[0098] table 5
[0099]
[0100] Prepare negative control and blank control according to Table 6.
[0101] Table 6
[0102] name Component concentration Loading volume (μL) negative control Human Embryonic Kidney Cell 293T Culture Medium 5×10 4 cell / mL
Embodiment 2
[0103] Example 2 The method of using the new coronavirus and pneumonia pathogenic bacteria detection kit
[0104] The method for using the detection kit of the present invention comprises the following steps:
[0105] (1) PCR reaction solution preparation
[0106] Take out nucleic acid amplification reaction solution A, nucleic acid amplification reaction solution B and enzyme mixture from the kit, thaw at room temperature, shake and mix well, according to the number of samples to be amplified N (including positive control, negative control and blank control) 7 and Table 8 respectively prepare the PCR reaction solution A tube and the PCR reaction solution B tube, mix thoroughly, centrifuge for a short time and dispense into PCR reaction tubes (plates) with a volume of 12.5 μL.
[0107] Table 7 Preparation of tube A of PCR reaction solution
[0108] component name Volume (μL) Reaction solution preparation Nucleic Acid Amplification Reaction Solution A 11 ...
Embodiment 3
[0121] Example 3 Sensitivity of the kit of the present invention for detection
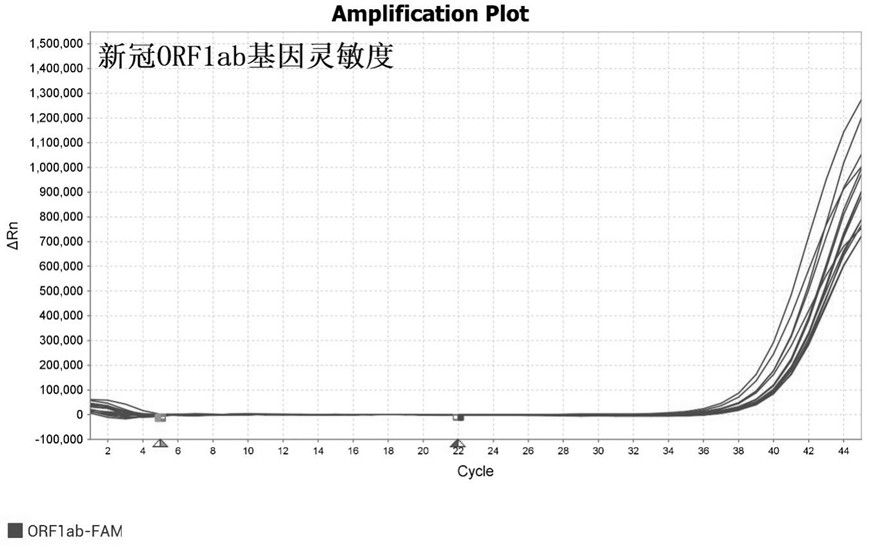
[0122] Using the kit of Example 1, detect the national standard product 2019-nCoV nucleic acid detection reagent national reference product (370099-202001), verify the detection sensitivity; the sensitivity reference product S concentration (stock solution) is 3 × 10 5 Copies / mL, jointly determined by digital PCR method. S using RNA / DNase-free deionized water for 1:3 dilution (2 parts water + 1 sample), 1:9, 1:27, 1:81, 1:243, 1:729, 1 :2187, 1:6561, 1:19683, 1:59049, and 1:177147 were marked as S1-S10 respectively, and were detected after nucleic acid extraction according to the kit instructions. According to the test results after serial dilution, the detection sensitivity of this kit to SARS-CoV-2 is 0.137 copies / μL (S6), such as Figure 3-Figure 4 shown, where image 3 Schematic diagram of the detection sensitivity of the novel coronavirus ORF1ab gene; Figure 4 It is a schematic diagram o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com