Compound antihypertensive tablet and preparation method thereof
A compound formula and tablet technology, which is applied in the field of medicine, can solve the problems such as the difficulty of stably controlling the blood pressure of elderly patients with hypertension, and achieve the effects of good in vitro dissolution, optimization of process parameters and reduction of losses.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
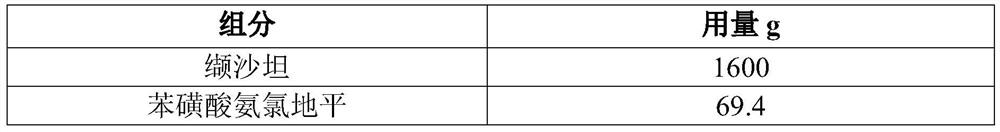
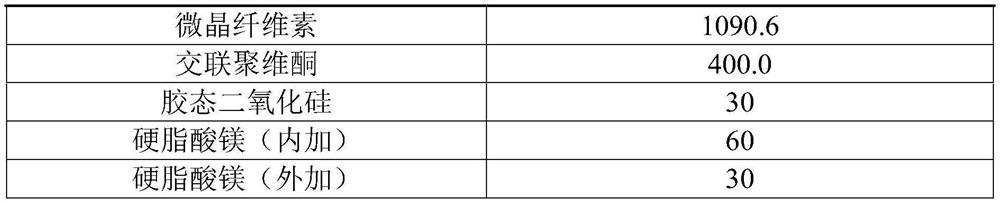
[0054] In this embodiment, 10,000 tablets of valsartan and amlodipine are prepared (each tablet contains 160 mg of valsartan, 5 mg of amlodipine, and the conversion factor of amlodipine and amlodipine besylate is 0.721), and its prescription is as follows in Table 1 Shown:
[0055] Table 1 Prescription of 10,000 valsartan and amlodipine tablets
[0056]
[0057]
[0058] The preparation method comprises the following steps:
[0059] The raw materials amlodipine besylate and colloidal silicon dioxide are respectively passed through a 40-mesh sieve, and the raw and auxiliary materials are weighed according to the prescription quantity. According to the order of addition: first add a part of microcrystalline cellulose (40% of the total mass), and then cross-link Povidone, amlodipine besylate, silicon dioxide, magnesium stearate (internal addition), valsartan, add in the multifunctional wet mixing granulator, stirring speed is 120rpm, and the cutting knife speed is 120rpm, ...
Embodiment 2
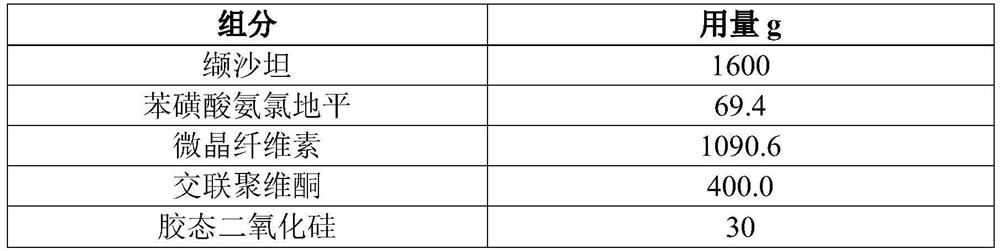
[0061] In this embodiment, 10,000 tablets of valsartan and amlodipine are prepared (each tablet contains 160 mg of valsartan, 5 mg of amlodipine, and the conversion factor of amlodipine and amlodipine besylate is 0.721), and its prescription is as follows in Table 2 Shown:
[0062] Table 2 Prescription of 10,000 Valsartan and Amlodipine Tablets
[0063]
[0064]
[0065] The preparation method comprises the following steps:
[0066] The preparation method is the same as that in Example 1 of the present invention, the difference is that the oil pressure is added, and the specific dry granulation parameters are as follows, the feeding speed is 40rpm, the roller is 12rpm, the oil pressure is 70bar, the granulation is 100rpm, and the roller gap is 0.6-0.7 mm for granulation, the specific particle size distribution is shown in Table 5.
Embodiment 3
[0068] In this embodiment, 100,000 valsartan and amlodipine tablets (each containing 160 mg of valsartan, 5 mg of amlodipine, and a conversion factor of 0.721 for amlodipine and amlodipine besylate) are prepared, and the prescription is as follows in Table 3 Shown:
[0069] Table 3 Prescription of 100,000 Valsartan and Amlodipine Tablets
[0070] components Dosagekg Valsartan 16.0 Amlodipine 0.694 microcrystalline cellulose 10.906 Crospovidone 4 colloidal silica 0.3 Magnesium stearate (added) 0.6 Magnesium Stearate (additional) 0.3
[0071] The preparation method comprises the following steps:
[0072] The preparation method is the same as that in Example 1 of the present invention, the difference is that the gap between the rollers has been increased in the investigation, and the specific dry granulation parameters are as follows, the setting is to increase the feeding speed to 50 rpm, the roller to 12 rpm, the oil pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com