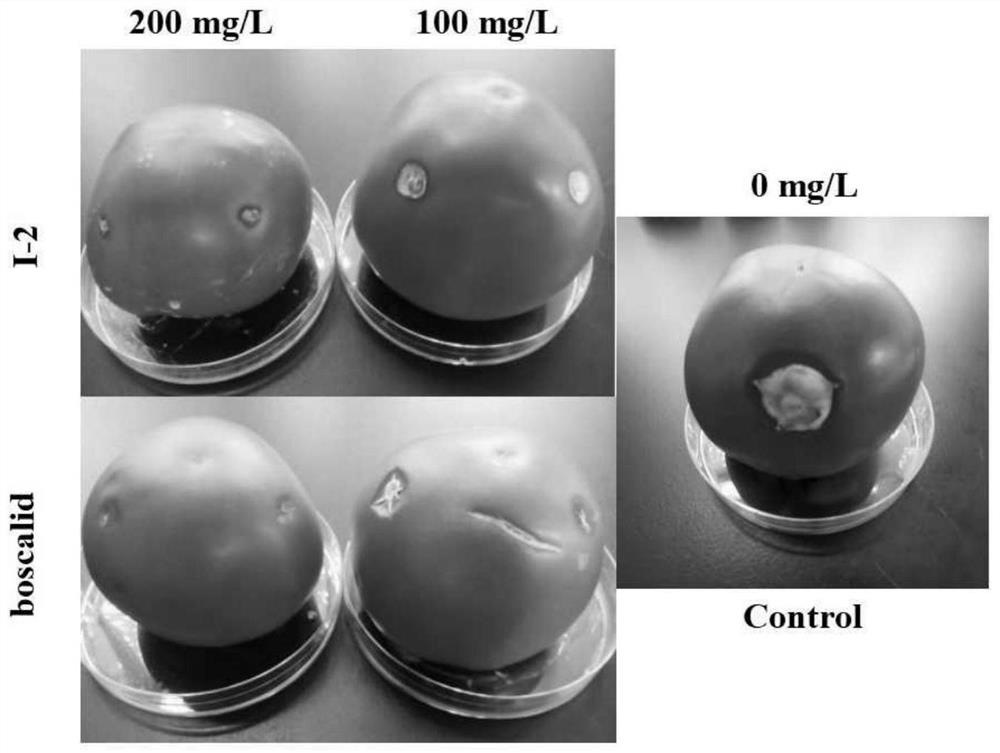
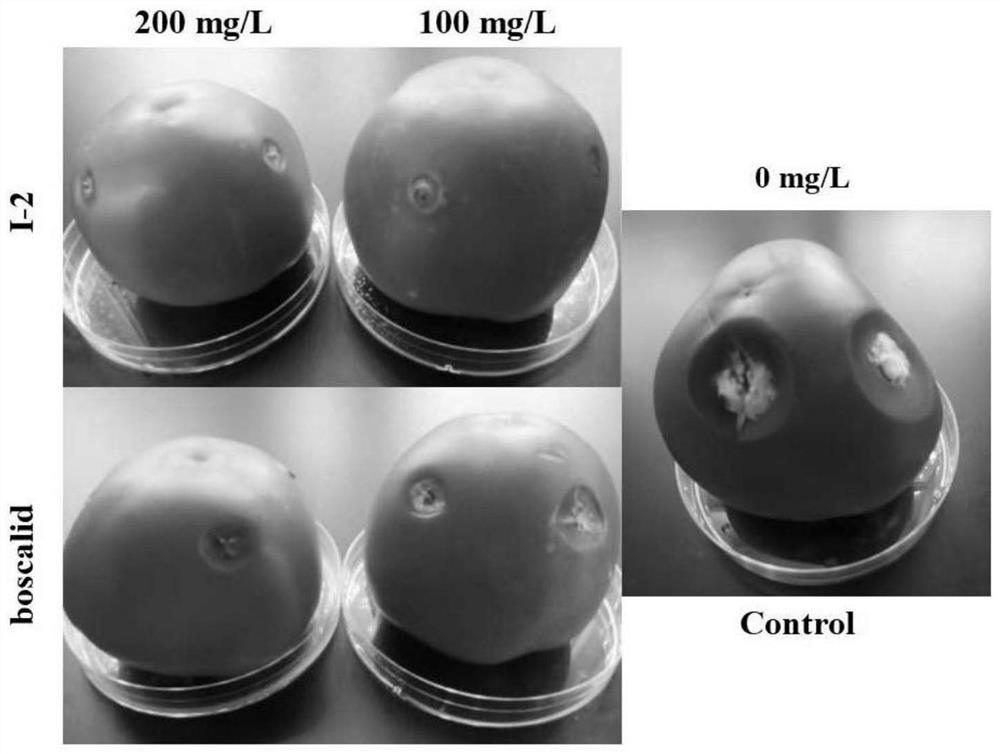
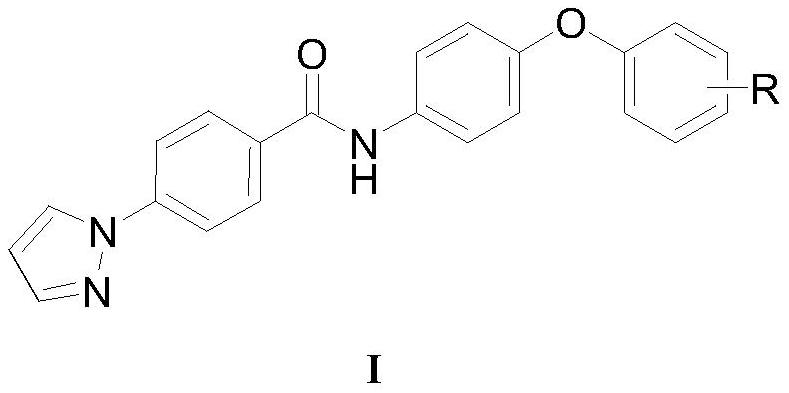
4-(1H-pyrazol-1-yl) biphenyl formamide compound containing diphenyl ether group and application thereof
A technology of biphenylcarboxamide and phenoxyaniline, which is applied in the field of pesticide synthesis, can solve the problems of abnormal fallen leaves, dead plants and the like, and achieves the effects of simple preparation method, easily available raw materials and novel molecular structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of 4-(1H-pyrazol-1-yl) biphenyl carboxamides containing diphenyl ether group:
[0034] 4-(1H-pyrazol-1-yl)benzonitrile (0.338g, 1.0mmol) was dissolved in 25mL (ethanol / water, volume ratio, 1:1), heated to 105°C for 16h under stirring, and the reaction solution was poured Pour into water, extract with ethyl acetate (30 mL), acidify the aqueous phase with 6.0 mol / L to pH = 5, precipitate a solid, filter with suction, and dry to give 4-(1H-pyrazol-1-yl)benzoic acid as a white solid, Yield 65%. 1 H NMR (600MHz, DMSO) δ13.13(s, 1H), 8.65(d, J=2.3Hz, 1H), 8.10(d, J=8.5Hz, 2H), 8.02(d, J=8.6Hz, 2H ),7.85(s,1H),6.63(s,1H). 13 C NMR (150MHz, DMSO) δ166.71, 142.73, 141.88, 130.94, 128.24, 128.15, 117.92, 108.63.
[0035]
[0036] Add 4-(1H-pyrazol-1-yl)benzoic acid (1.2mmol), 4-phenoxyaniline (1.0mmol), 1-ethyl-(3-dimethylaminopropyl) into a 25mL one-mouth bottle Carbodiimide hydrochloride (EDCI, 1.2mmol), 4-dimethylaminopyridine (DMAP, 0.2mmol) and CH 2 Cl 2...
Embodiment 2
[0038]
[0039] Add 4-(1H-pyrazol-1-yl)benzoic acid (1.2mmol), 4-(4-chlorophenoxy)aniline (1.0mmol), 1-ethyl-(3-dimethyl Aminopropyl) carbodiimide hydrochloride (EDCI, 1.2mmol), 4-dimethylaminopyridine (DMAP, 0.2mmol) and CH 2 Cl 2 (10mL), stirred at 25°C for 2-5h. TLC monitored the complete reaction of the raw materials, and the reaction solution was filtered with suction. The obtained solid was purified by beating with petroleum ether to obtain compound I-2, a white solid, m.p.243-245°C, and the yield was 73%. 1 H NMR (600MHz, DMSO) δ10.83(s, 1H), 7.98(dd, J1=7.8Hz, J2=1.5Hz, 2H), 7.84(d, J=9.0Hz, 2H), 7.60-7.55(m ,4H),7.50(s,1H),7.44-7.41(m,2H),7.10-7.08(m,2H),7.03-7.02(m,2H).13C NMR(150MHz,DMSO)δ164.46,156.33,151.69 ,141.80,141.69,135.40,132.00,129.80,129.28,128.16,126.72,122.19,119.54,119.47,117.71,108.47.ESI-HRMS:m / zcalcd.for C22H17ClN3O2[M+0H.1f.1f.
Embodiment 3
[0041]
[0042] Add 4-(1H-pyrazol-1-yl)benzoic acid (1.2mmol), 4-(2-chlorophenoxy)aniline (1.0mmol), 1-ethyl-(3-dimethyl Aminopropyl) carbodiimide hydrochloride (EDCI, 1.2mmol), 4-dimethylaminopyridine (DMAP, 0.2mmol) and CH 2 Cl 2 (10mL), stirred at 25°C for 2-5h. TLC monitored the complete reaction of the raw materials, and the reaction solution was filtered with suction. The obtained solid was purified by beating with petroleum ether to obtain compound I-3, a white solid, m.p.189-190°C, and the yield was 89%. 1 H NMR (600MHz, DMSO) δ10.32(s, 1H), 8.65(d, J=2.5Hz, 1H), 8.10(d, J=8.8Hz, 2H), 8.02(d, J=8.8Hz, 2H ),7.82-7.79(m,3H),7.60(dd,J1=8.0Hz,J2=1.5Hz,1H),7.38-7.35(m,1H),7.22-7.19(m,1H),7.06(dd, J 1 =8.2Hz,J 2 =1.4Hz,1H),7.01-6.99(m,2H),6.62-6.61(m,1H).13C NMR(150MHz,DMSO)δ164.65,161.01,154.87,152.12,141.69,130.70,129.21,128.77,128.14, 125.02, 122.16, 122.05, 120.34, 118.10, 117.70, 108.46. ESI-HRMS: m / zcalcd. for C22H17ClN3O2[M+H]+: 390.1009; found 390.1011.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com