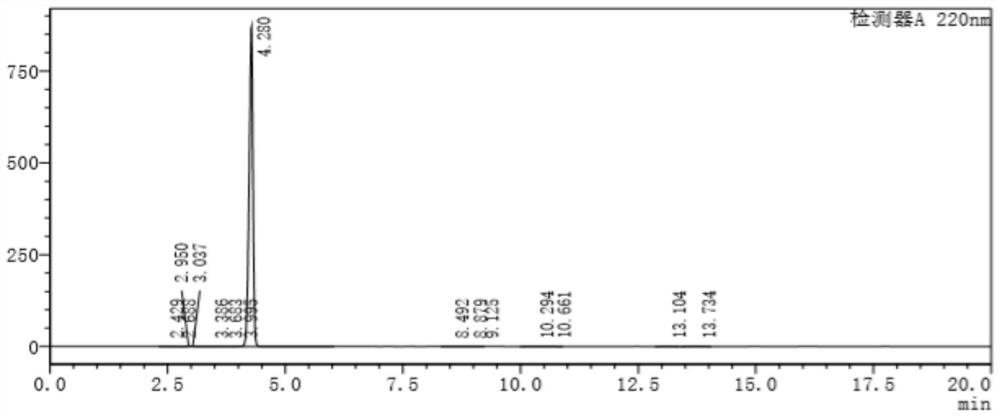
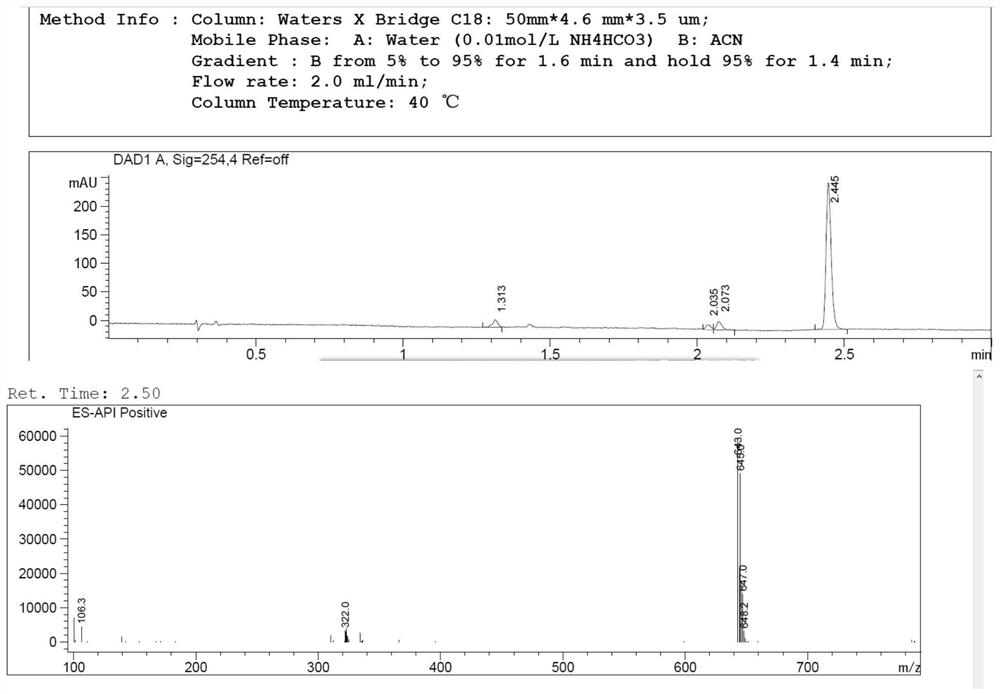
Clopidogrel bisulfate impurity A and preparation method thereof
A technology of clopidogrel bisulfate and hydroxymethyl clopidogrel, applied in organic chemistry methods, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A kind of preparation method of clopidogrel bisulfate impurity A provided by the embodiment of the present invention comprises the following steps:
[0047] 1. Preparation of hydroxymethylclopidogrel impurity Z1:
[0048] 1.1 Take an appropriate amount of hydrochloric acid and a 250ml three-necked flask, take an appropriate amount of concentrated sulfuric acid and add it to a constant pressure dropping funnel, and slowly add it dropwise to the hydrochloric acid. In the three-necked bottle of methyl chloride, absorb to saturation, seal it for use;
[0049] 1.2 Take another 500ml three-neck bottle, accurately weigh the condensate hydrochloride (38.72g, 1eq), paraformaldehyde (19.95g, 6.0eq), formic acid (1.61g, 0.31eq), configure a thermometer, magnetic stirring device, reflux device, add the methylene chloride solution that absorbs hydrogen chloride gas to saturation in 1.1, seal the reaction, reflux at 40°C, reflux temperature is 37°C, and reflux time is 48 hours, stop...
Embodiment 2
[0058] A kind of preparation method of clopidogrel bisulfate impurity A provided by the embodiment of the present invention comprises the following steps:
[0059] 1. Preparation of hydroxymethylclopidogrel impurity Z1:
[0060] 1.1 Take an appropriate amount of hydrochloric acid and a 250ml three-necked flask, take an appropriate amount of concentrated sulfuric acid and add it to a constant pressure dropping funnel, and slowly add it dropwise to the hydrochloric acid. In the three-necked bottle of methyl chloride, absorb to saturation, seal it for use;
[0061] 1.2 Take another 500ml three-neck bottle, accurately weigh the condensate hydrochloride (38.72g, 1eq), paraformaldehyde (19.95g, 6.0eq), formic acid (1.61g, 0.31eq), configure a thermometer, magnetic stirring device, reflux device, add the methylene chloride solution that absorbs hydrogen chloride gas to saturation in 1.1, seal the reaction, reflux at 40°C, reflux temperature is 37°C, and reflux time is 48 hours, stop...
Embodiment 3
[0066] A kind of preparation method of clopidogrel bisulfate impurity A provided by the embodiment of the present invention comprises the following steps:
[0067] 1. Preparation of hydroxymethylclopidogrel impurity Z1:
[0068] 1.1 Take an appropriate amount of hydrochloric acid and a 250ml three-necked flask, take an appropriate amount of concentrated sulfuric acid and add it to a constant pressure dropping funnel, and slowly add it dropwise to the hydrochloric acid. In the three-necked bottle of methyl chloride, absorb to saturation, seal it for use;
[0069] 1.2 Take another 500ml three-neck bottle, accurately weigh the condensate hydrochloride (38.72g, 1eq), paraformaldehyde (19.95g, 6.0eq), formic acid (1.61g, 0.31eq), configure a thermometer, magnetic stirring device, reflux device, add the methylene chloride solution that absorbs hydrogen chloride gas to saturation in 1.1, seal the reaction, reflux at 40°C, reflux temperature is 37°C, and reflux time is 48 hours, stop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com