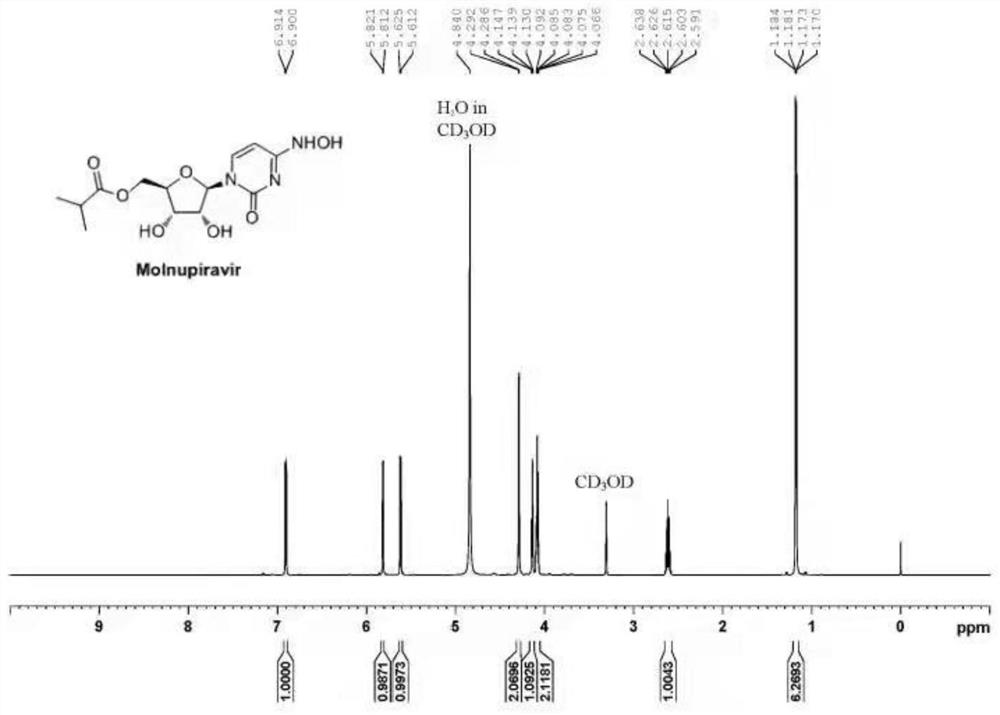
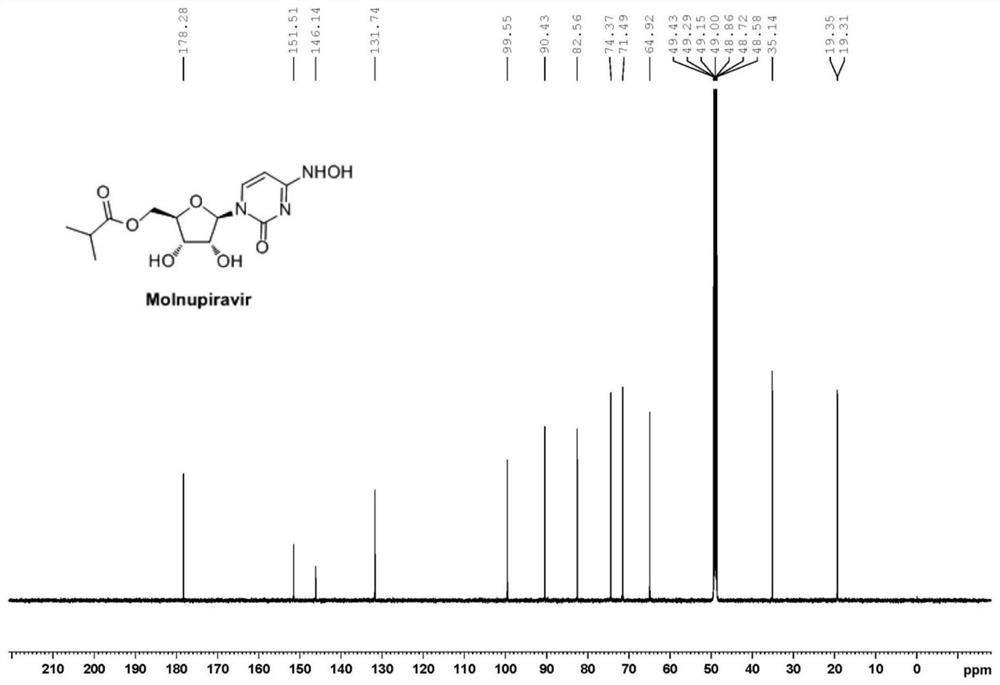
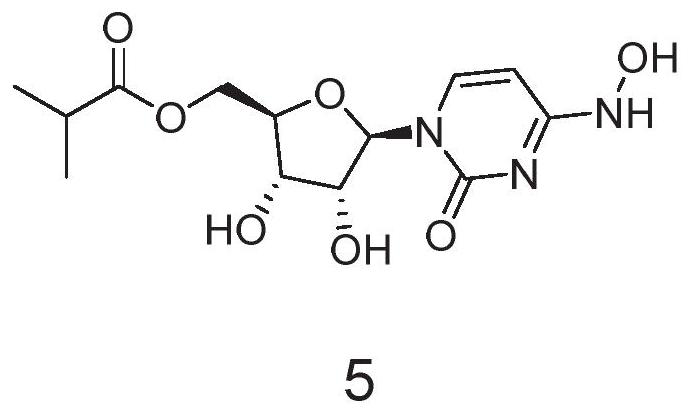
Preparation method of Molnupiravir
A compound and reaction technology, applied in the field of medicine, can solve the problems of the product monabiravir, which is difficult to improve the quality, unfavorable for product separation and purification, and the acetonide protecting group is easy to fall off. friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1.1 Preparation of compound 4:
[0057] Reaction A: Add compound 1 (cytidine) (5g, 20mmol), acetone (80mL) and 2,2-dimethoxypropane (10mL) in a reaction flask, add concentrated sulfuric acid (1mL, 20mmol) dropwise at 0°C ), rose to room temperature and reacted for about 10 hours until compound 1 was completely reacted. 20% aqueous sodium hydroxide solution was added dropwise at 0°C to adjust the pH of the system to 8-10. Acetone was distilled under reduced pressure to no distillate. Acetonitrile (50 mL) was added to the residue, heated to above 60° C., stirred for 30 minutes, and filtered. Acetonitrile (20 mL) was added to the filter cake, heated to 60° C. and stirred for 30 minutes, and filtered to obtain a filtrate. The filtrates were combined, dried over anhydrous sodium sulfate, and filtered to obtain an acetonitrile solution of compound 2, which was directly used in the next reaction.
[0058] Reaction B: Triethylamine (6.1 g, 60 mmol) was added to the acetonit...
Embodiment 2
[0063] 2.1 Preparation of Compound 4:
[0064] Reaction A: At room temperature, add cytidine (150kg, 1.0eq), 2,2-dimethoxypropane (256kg, 4.0eq) and acetone (1950kg) into the reactor, and stir evenly. Cool down to below 0°C, and add concentrated sulfuric acid dropwise. Raise the temperature to room temperature and react for 10 hours until the cytidine reaction is complete, then cool down to 0°C, and add triethylamine (about 130kg) dropwise until the system pH=8-10. Concentrate under reduced pressure until there is no distillate, add acetonitrile (1500kg) and stir evenly to obtain an acetonitrile solution of compound 2, which is directly used in the next reaction.
[0065] Reaction B: At room temperature, add triethylamine (187kg, 3.0eq) and 4-dimethylaminopyridine (7.5kg, 0.1eq) to the acetonitrile solution of compound 2 in the previous step, and stir well. The temperature was lowered to 0°C, and isobutyryl chloride (164kg, 2.5eq) was added dropwise. Keep warm at 5°C for ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com