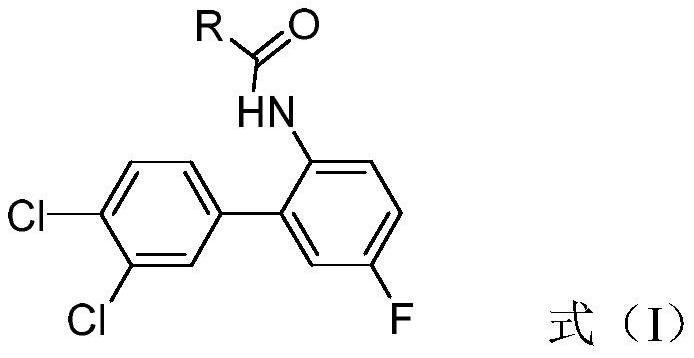
Biphenyl heterocyclic compound as well as synthesis method and application thereof
A technology for a heterocyclic compound and a synthesis method, which is applied in the synthesis and antifouling fields of biphenyl heterocyclic compounds, can solve the problems of aggravating the corrosion and damage of marine engineering materials, endangering the marine ecological environment, reducing the performance of marine facilities, etc., so as to avoid artificial The effect of synthesis, price advantage, simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
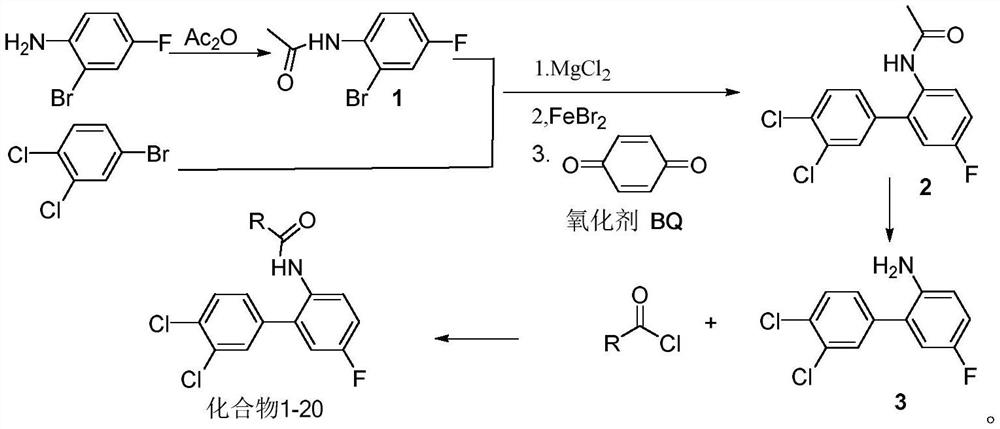
[0030] The synthesis process, steps are as follows:
[0031] (1) Add 20mL of dichloroethane and 5.2g (0.03mol) of 2-bromo-4-fluoroaniline into a 250mL three-neck flask, add 3.1g (0.03mol) of acetic anhydride dropwise at room temperature, and complete the dropwise addition in 20 minutes. Sampling was carried out to obtain intermediate 1 (acetanilide) with a yield of 99%.
[0032] (2) At room temperature, the compound 2-bromo-4-fluoroacetanilide (3.7g, 16mmol) and 3,4-dichlorobromobenzene (3.6g, 16mmol) were dissolved in THF (50mL), electromagnetically stirred, and then Magnesium chloride reagent (4.9 g, 24 mmol) was added dropwise and reacted at 50° C. for 2 h. In another three-necked flask, FeBr will be added 2 (1.16g, 10mmol) in THF (50mL), after stirring for 30min, the above-mentioned mixture was added to the solution, and after continuing to react for 20min, the oxidant BQ (2.16g, 20mmol) was added and reacted at room temperature for 30min, and the reaction solution was...
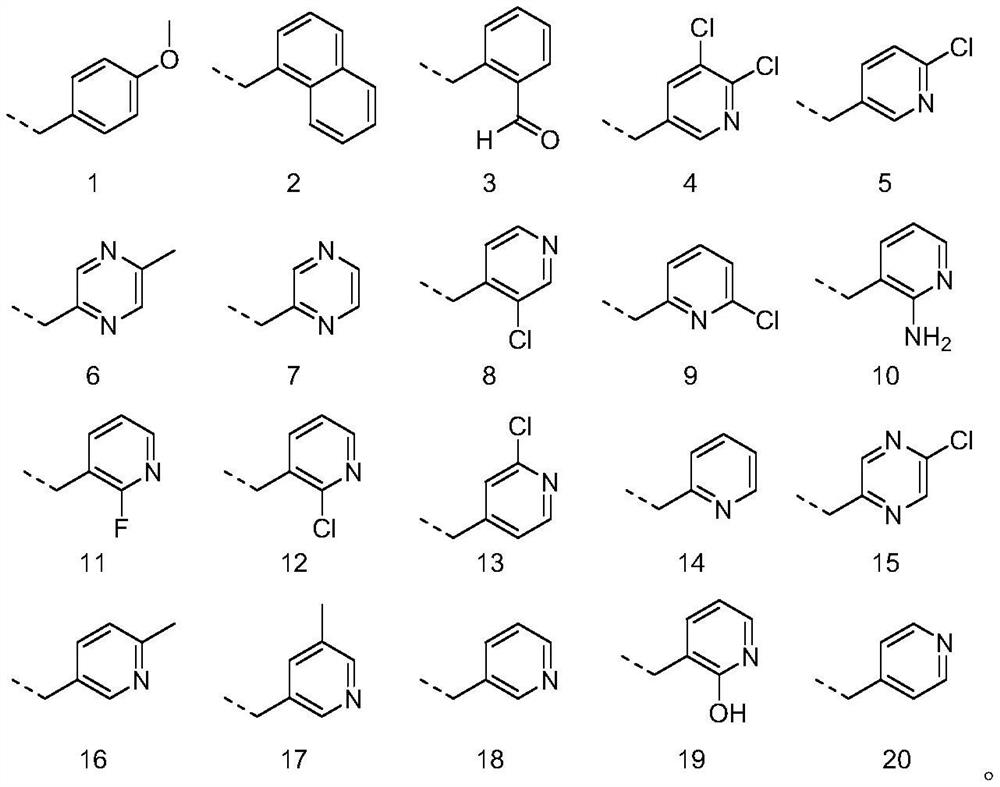
Embodiment 2-20
[0040] Table 1 Physicochemical properties of antifouling compounds
[0041]
Embodiment 2
[0043] Identification
[0044] Compound 2.1 H-NMR (400MHz,) δ8.30–8.15 (m, 2H), 8.39–8.09 (m, 2H), 7.93 (d, J=8.1Hz, 1H), 8.02–7.76 (m, 2H), 7.87 ( dd,J=5.9,3.2Hz,1H),8.79–6.57(m,16H),7.50(ddd,J=25.6,11.8,5.5Hz,8H),7.50(ddd,J=25.6,11.8,5.5Hz, 7H), 7.22–7.13(m,1H), 7.38–7.14(m,3H), 7.14–6.94(m,1H), 7.07–6.99(m,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com