Chenodeoxycholic acid phenylethylamine salt, preparation method and refining method thereof, and chenodeoxycholic acid preparation method
A technology for chenodeoxycholic acid and phenethylamine salts, which is applied in the preparation of amino compounds, steroids, organic chemistry, etc. Remove bile acid impurities and other problems, and achieve the effect of ammonium salt with white color, high content and good impurity removal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
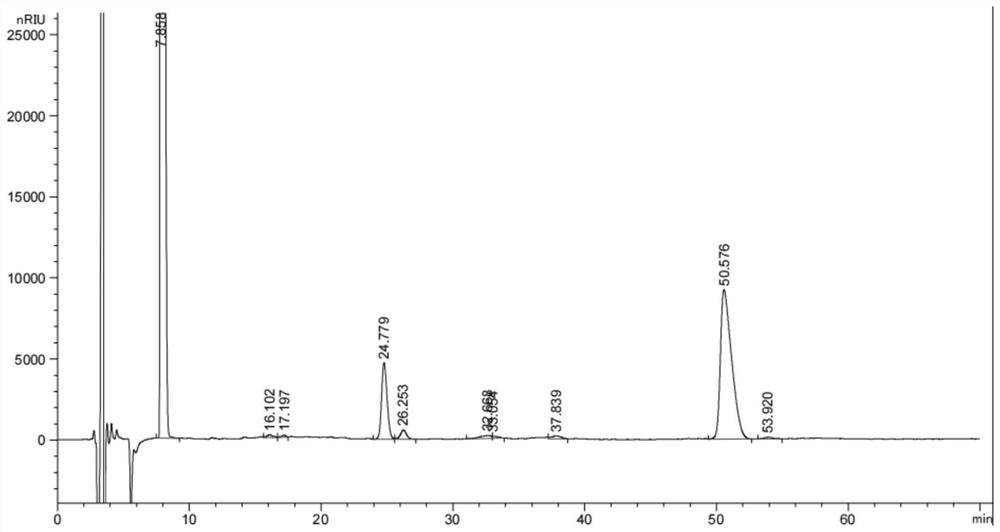
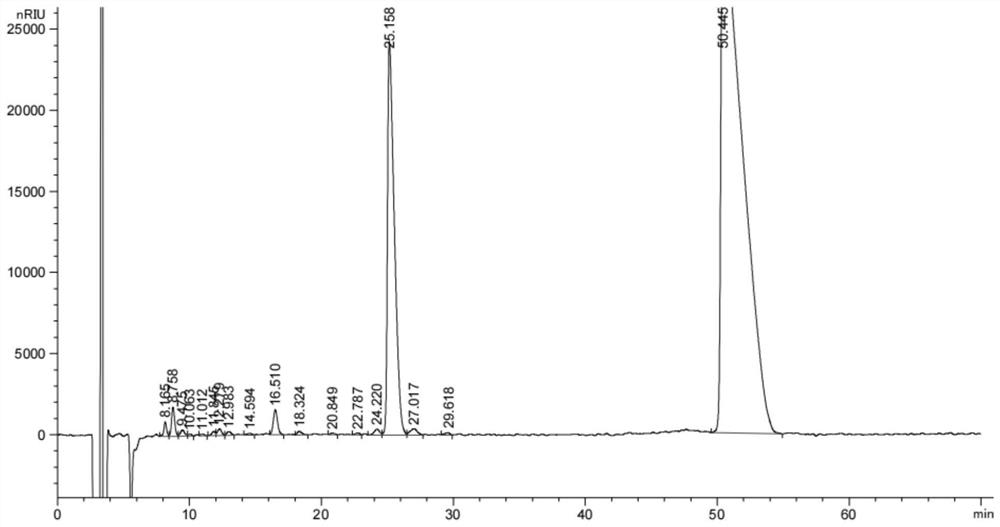
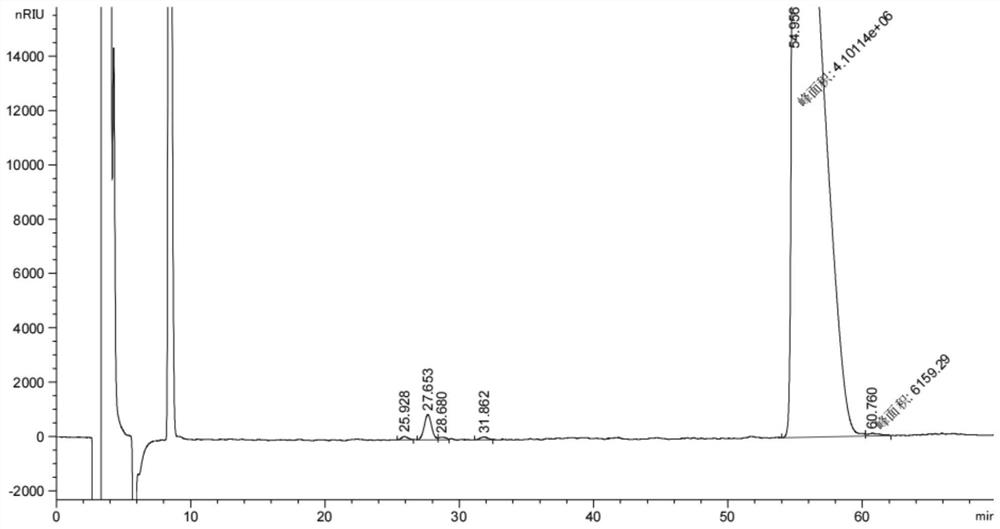
Image
Examples
Embodiment 1
[0039] S1 goose oxygen-oxaglean salt (referred to as CD salt) crude product
[0040] The reaction kettle is added to 200kg (2 times the chilloballine weight), 50 kg of water (0.5 times the weight of the chillion), add the chicken bile cream 100 kg; stir up to 60-65 ° C, stir, and regulate pH to 1 ~ 6, solve material; resetting separation; the aqueous layer 50 kg (0.5 times chollery paste is 0.5 times), stirring at 50-55 ° C for 5 minutes, sedation; buteate Layer, 100 kg of water, stirred for 5 minutes, reset separately, butyl acetate (ie, a goose deoxycholic acid solution) weigh, sampling test, measure CDCA (goose deoxycholic acid referred to as) and CA (gallbladder Acid referred to.
[0041] The butyl acetate layer was added to 17.5 kilograms of A-phenylamine, concentrated from no acetate butyl acetate at 60-90 ° C, and the temperature to 60-65 ° C was adjusted, and about 12.5 kg of A-phenylamine, control pH 8-9, The addition of A-phenylamine was accurate in pH, and the temperature...
Embodiment 2
[0067] S1 goose oxygen-oxaglean salt (referred to as CD salt) crude product
[0068] The reaction kettle was added to 200kg (2 times the chilloconoline weight), 50 kg of water was added (0.5 times the choliobes), and the chicken bold paste was added to 100 kg. The temperature is heated to 60-65 ° C, stirred and regulates the pH to 5.0, the material; the sedation is separated; the aqueous layer is 50 kg (0.5 times the chills of the chilli), and the mixture is stirred at 50-55 ° C for 5 minutes. Static separation. Combined with both acetate layer, 100 kg of water, stirred for 5 minutes, resetting the separation, the acetate layer weighing, sampling the test, the CDCA (Goose deoxycholic acid referred to as) and the CA (Cholic acid abbrevantation) content .
[0069] The butyl acetate layer was added to 12 kg of tert-butlamine, stirred at 50-65 ° C for 1 hour, cooled to 25-30 ° C, continued for 1 hour, centrifugally, washing the filter cake with a acetate, deserted oxychloric tert-buta...
Embodiment 3
[0076] The reaction kettle was added to 200kg (2 times the chilloconoline weight), 50 kg of water was added (0.5 times the choliobes), and the chicken bold paste was added to 100 kg. The temperature is heated to 60-65 ° C, stirred and regulates the pH to 1 to 6, the material; the sedation is separated; the aqueous layer is 50 kg (0.5 times the chills of chilli paste), and stirred in 50-55 ° C. 5 Minutes, resetting liquid. Combined with both a acetate layer, 100 kg of water was extracted, stirred for 5 minutes, resetting the separation, and obtained the butyl acetate layer A.
[0077] Some salt organic base screening experiments are as follows:
[0078] A-phenylamine (R - (+) - A-phenylamine: s - (+) - A-athylamine = 1: 1 hoe body): buteate layer A 60-90 ° C concentrated The butyl acetate is 140 liters, adjusting the temperature to 60-65 ° C, adding A-phenylamine to pH to 9. The incubation was stirred for 20-30 minutes, then cooled to 25-30 ° C, the temperature was 30 ° C, stirring...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com