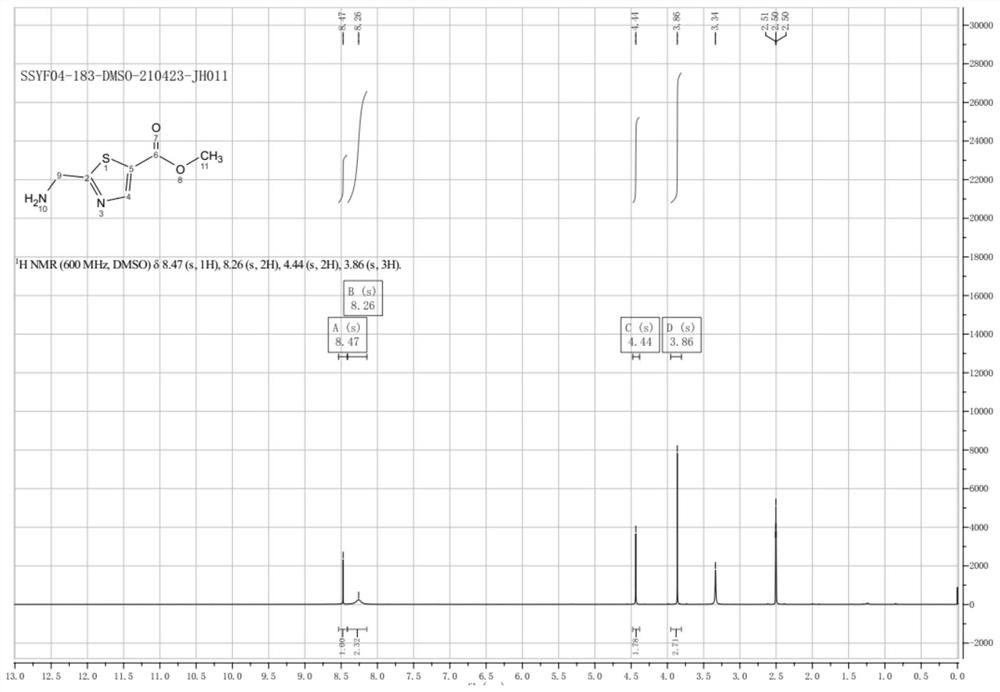
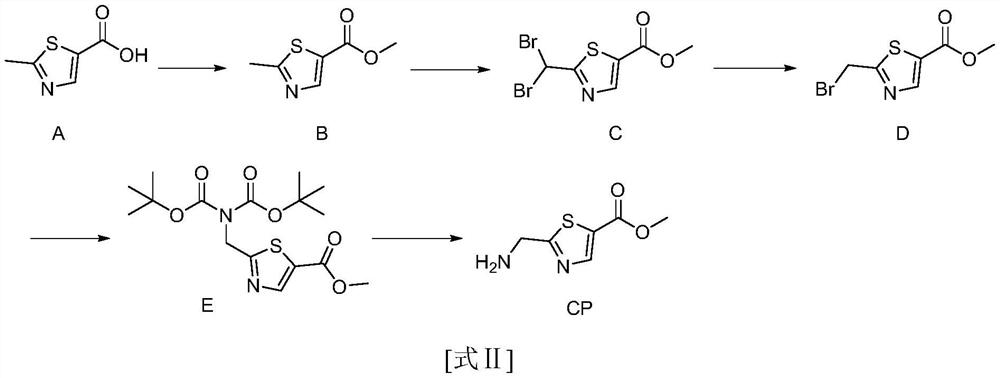
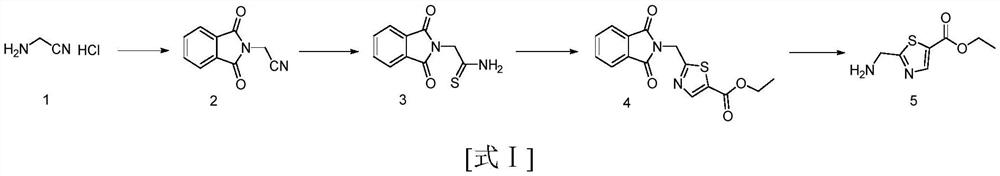
Synthesis method of 2-(aminomethyl)-1, 3-thiazole-5-carboxylic acid methyl ester
A technology of methyl carboxylate and aminomethyl, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of unsuitable overall process, unavailable ring-closing raw materials, and strong taste of reagents, so as to improve consistency, reduce production costs, and protect the environment. The effect of little pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This example prepares methyl 2-(aminomethyl)-1,3-thiazole-5-carboxylate, and the synthetic route is as follows:
[0041]
[0042] Specific steps are as follows:
[0043] Step 1: Add compound A (100g, 0.70mol, 1eq) to 1000ml methanol, then add concentrated sulfuric acid (100g, 1mol, 1.4eq), heat to reflux overnight, TLC detection, the reaction is complete, pour into ice water, ethyl acetate After extraction, backwashing with sodium bicarbonate solution and spinning to dry, 106 g of compound B was obtained as a brown liquid with a yield of 91.8% and a purity of 95%.
[0044] Step 2: Add compound B (106g, 0.64mol, 1eq) to 1000ml of carbon tetrachloride, then add N-bromosuccinimide (214g, 2.12mol, 2.5eq), heat to reflux, batch by batch Add azobisisobutyronitrile (69.36, 0.42mol, 0.66eq), continue to reflux overnight after addition, TLC detects that the reaction is complete, cool and filter, wash the filter cake with dichloromethane, combine the organic phases to obtain ...
Embodiment 2
[0050] This example prepares methyl 2-(aminomethyl)-1,3-thiazole-5-carboxylate, and the specific steps are as follows:
[0051] Step 1: Add compound A (10g, 70mmol, 1eq) to 100ml of methanol, then add concentrated sulfuric acid (10g, 100mmol, 1.4eq), heat to reflux overnight, TLC detection, the reaction is complete, pour into ice water, and extract with ethyl acetate , backwashed with sodium bicarbonate solution and spin-dried to obtain 9.42 g of compound B as a brown liquid, with a yield of 78% and a purity of 91%.
[0052] Step 2: Add compound B (9.42g, 55mmol, 1eq) to 100ml of carbon tetrachloride, then add N-bromosuccinimide (19.58g, 110mmol, 2.0eq), heat to reflux, batch by batch Add azobisisobutyronitrile (2.71g, 16.5mmol, 0.3eq), continue to reflux overnight after the addition, TLC detection, the reaction is complete, cool and filter, wash the filter cake with dichloromethane, combine the organic phases to obtain 150ml of compound C solution, The quantitative content i...
Embodiment 3
[0057] This example prepares methyl 2-(aminomethyl)-1,3-thiazole-5-carboxylate, and the specific steps are as follows:
[0058] Step 1: Add compound A (10g, 70mmol, 1eq) to 100ml of methanol, then add concentrated sulfuric acid (5g, 50mmol, 0.7eq), heat to reflux overnight, TLC detection, the reaction is complete, pour into ice water, and extract with ethyl acetate , backwashed with sodium bicarbonate solution and spin-dried to obtain 6.2 g of compound B as a brown liquid with a yield of 50% and a purity of 89%.
[0059] Step 2: Add compound B (6.2g, 35mmol, 1eq) to 60ml of carbon tetrachloride, then add dibromohydantoin (22.02g, 77mmol, 2.2eq), heat to reflux, and add azobisiso Butyronitrile (3.79g, 23.1mmol, 0.66eq), continued to reflux overnight after addition. According to TLC detection, the reaction was complete, cooled and filtered, the filter cake was washed with dichloromethane, and the organic phases were combined to obtain 90 ml of compound C solution with a quantit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com