Preparation method of chlorpheniramine maleate impurity
A kind of chlorpheniramine acid, impurity technology, is applied in the preparation field of chlorpheniramine maleate impurity, can solve the problems such as shortage of reference substance, few by-products, etc., achieves convenient operation, few by-products, and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of intermediate formula 3 compound
[0036] Put a 250mL three-necked bottle with a thermometer and a constant pressure funnel, seal it and replace it with N 2 , then add 30mL of anhydrous tetrahydrofuran and ethyl p-chlorophenylacetate (Compound 1) (6.00 g, 30.20 mmol, 1.0 eq), after cooling to -20°C, slowly add 1.0M bistrimethylsilylamine under stirring Lithium tetrahydrofuran solution (33.20ml, 33.20 mmol, 1.1 eq), after the addition was completed, the stirring was continued at -20°C for 30 minutes, and finally 6-fluoro-2-chloropyridine (compound 2) (11.92 g, 90.6mmol, 3.0 eq), after the addition was complete, return to room temperature and react for 4h, LC-Ms showed that the raw material was completely consumed. Slowly add 20mL of water in ice bath to quench the excess lithium bistrimethylsilylamide, remove tetrahydrofuran by rotary evaporation, then extract with 40mL ethyl acetate for 3 times, combine the organic layers, spin dry, and ...
Embodiment 2
[0037] Embodiment 2: the preparation of intermediate formula 4 compound
[0038] Intermediate 3 (5.50 g, 17.80 mmol, 1.0 eq), LiOH (4.35 g, 178.00 mmol, 10.0 eq), 30 mL of methanol and 30 mL of water were successively added into a 100 mL flask, and the temperature was raised to 100 °C under stirring, and reacted for 12 h. After LC-Ms showed that the reaction was complete, 20 mL of water was added to the system, methanol was removed by rotary evaporation, and then the organic layers were combined after extraction with 50 mL of ethyl acetate for 3 times and washed with anhydrous Na 2 SO 4 After drying, suction filtration, the filtrate was spin-dried to obtain intermediate 4 (3.99 g, 95% yield), light yellow liquid, R f =0.3(DCM:PE=1:2).
Embodiment 3
[0039] Embodiment 3: the preparation of chlorpheniramine maleate impurity
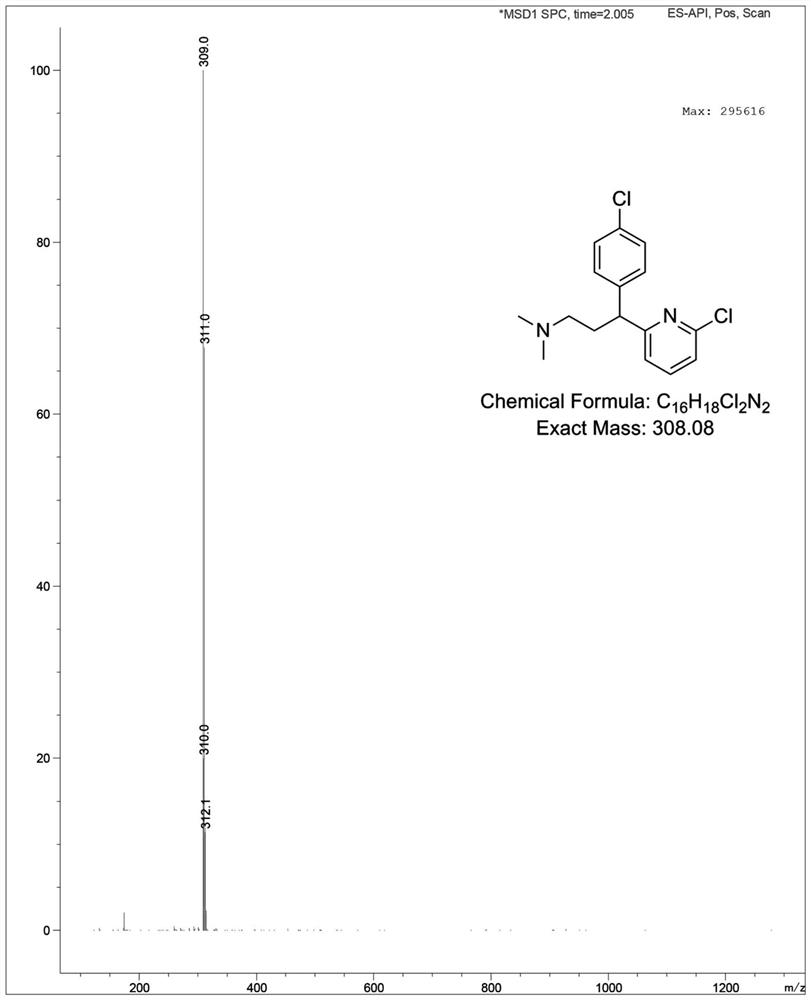
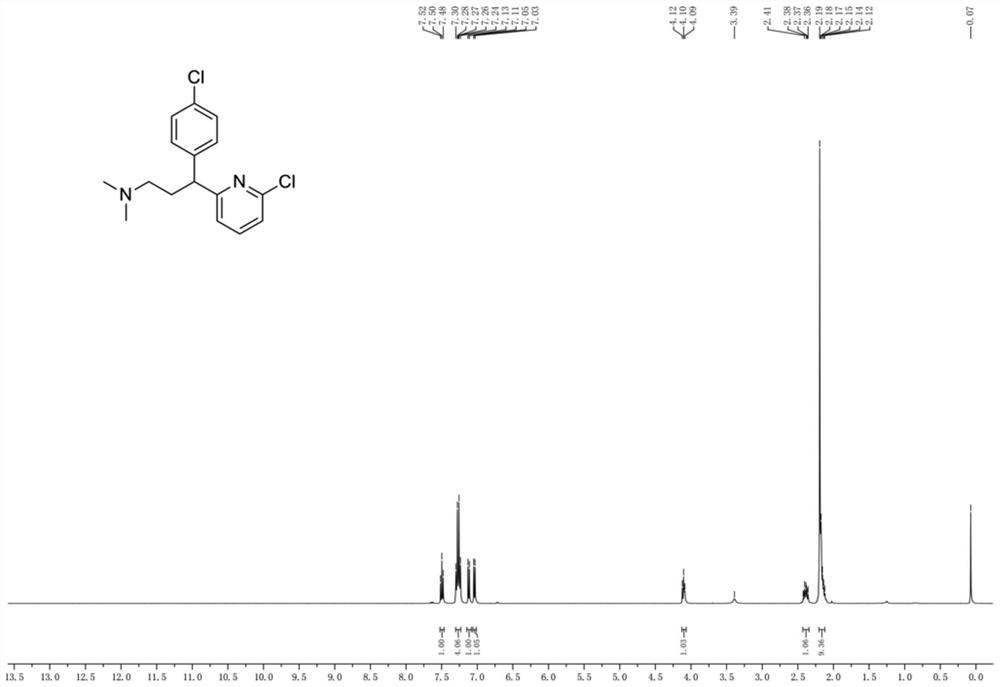
[0040] Put a 100mL three-necked bottle with a thermometer and a constant pressure funnel, seal it, and replace it with N 2 , then add 30mL of anhydrous tetrahydrofuran and intermediate 4 (2.98 g, 12.57 mmol, 1.0 eq), place in an ice bath and stir to dissolve, then slowly add 1.0M bistrimethylsilylamide lithium tetrahydrofuran solution, the addition is complete Continue to stir under ice bath for 30 minutes; then add N,N-dimethylaminobromoethane (2.19 g, 14.38 mmol, 1.1 eq), and continue to react under ice bath for 2 hours after the addition, LC- Ms shows complete consumption of starting material. Slowly add 10 mL of water in an ice bath to quench the excess lithium bistrimethylsilylamide , THF was removed by rotary evaporation, and then the organic layers were combined after being extracted 3 times with 50 mL ethyl acetate, and after being spin-dried, column chromatography (DCM:PE=1:1→DCM:MeOH=10:1 g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com