Whitmania pigra anticoagulant factor XIa polypeptide and application thereof
An antithrombotic drug and amino acid technology, applied in the field of peptides, can solve problems such as undiscovered, and achieve the effect of strong activity in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Discovery of WPK series polypeptides
[0033] Take the wide-bodied leech salivary gland, use magnetic beads to enrich mRNA, fragment it, synthesize the first strand and the second strand of cDNA with random primers, use QIAQuickPCR kit to purify, recover the target fragment by agarose gel electrophoresis, and amplify by PCR. Complete library construction. Illumina Hiseq 4000 was used for sequencing, and Trinity (version 2.4.0) was used for de novo analysis of transcriptome without reference transcriptome, Blast annotation and Pfam analysis, and 5 sequences were searched with "Kunitz" as the keyword, named WPK1-5. The sequence information is as follows:
[0034]
Embodiment 2
[0035] Example 2: Inhibitory effect of WPK series polypeptides on blood coagulation factor XIa
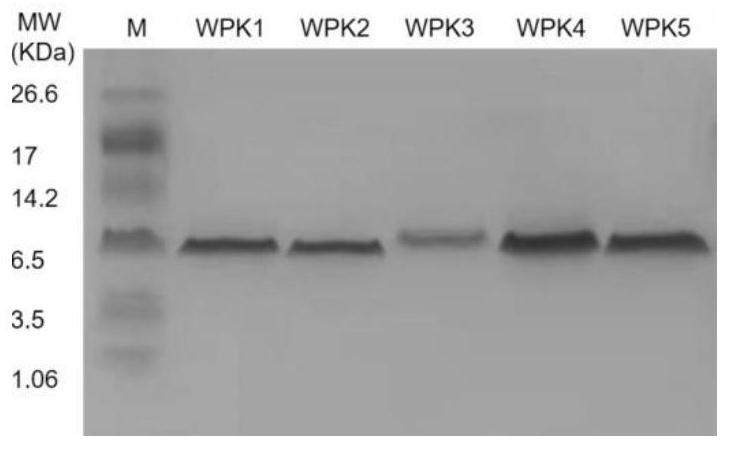
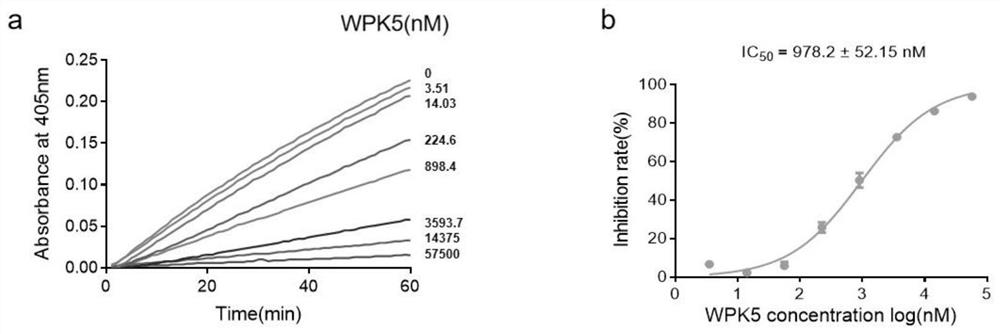
[0036] Construction of recombinant plasmid pPIC9k / WPK: Cloning the WPK1-5 sequence into pPIC9k using seamless cloning (cloning site NotI), linearized with SacI and electroporated into Pichia pastoris GS115, positive transformant colonies were identified by PCR and sequencing , Pick positive transformant colonies into the growth medium BMGY, 28.5 ° C, 220r / min shaking culture, when the OD600 of the bacterial solution is around 4-6, centrifuge at 4000r / min for 5min, and transfer the bacteria into the expression medium BMMY , add a final concentration of 1% methanol to induce, centrifuge to collect the supernatant after 72h, and obtain the WPK1-5 target protein through purification ( figure 1 ). To test the inhibitory activity of WPK series peptides on blood coagulation factor XIa, pipette 100 μL FXIa (1nM) and 50 μL WPK1-5 (10 μM) and mix evenly in a 96-well plate, incubate at 37°C ...
Embodiment 3
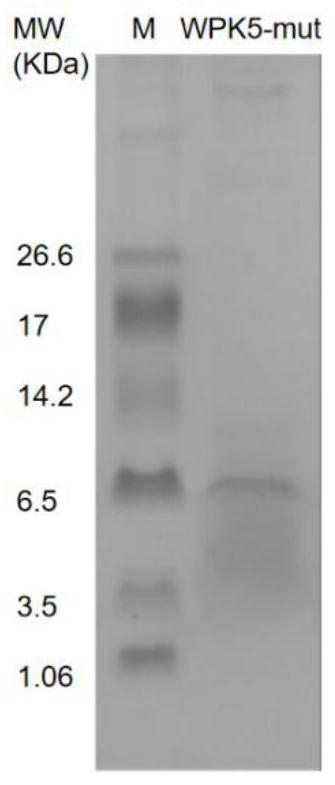
[0039] Example 3: The polypeptide mutant WPK5-mut sequence provided by the present invention and its comparison with the PN2KPI sequence
[0040] PN2KPI through Loop1( 11 TGPCRAMISR 20 ) and Loop2 ( 34FYGGC 38 ) extensively interacts with the catalytic domain of XIa and exhibits good inhibitory activity against coagulation factor XIa. In this embodiment, in order to further improve the inhibitory activity of WPK5 on blood coagulation factor XIa, the method of loop replacement is adopted, based on the new Kunitz skeleton provided by WPK5, two loops of PN2KPI ( 11 TGPCRAMISR 20 , 34 FYGGC 38 ) for WPK-5 two loops ( 11 TGPCRSNLER 20 , 34 wxya 38 ) to replace. And the amino acid sequence analysis of WPK5-mut and PN2KPI showed that the similarity was 60.78%.
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com