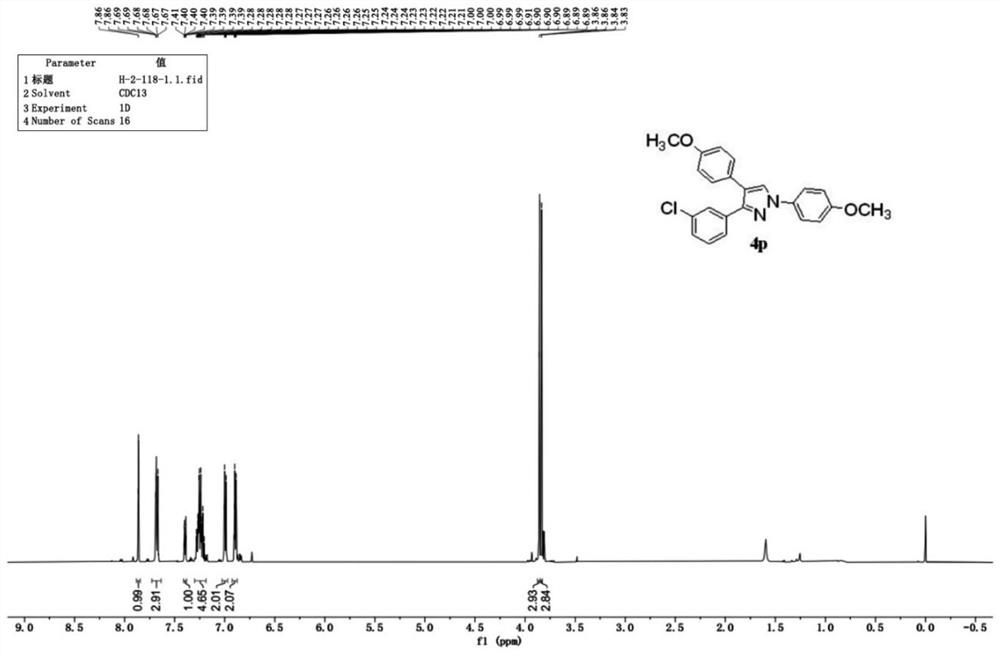
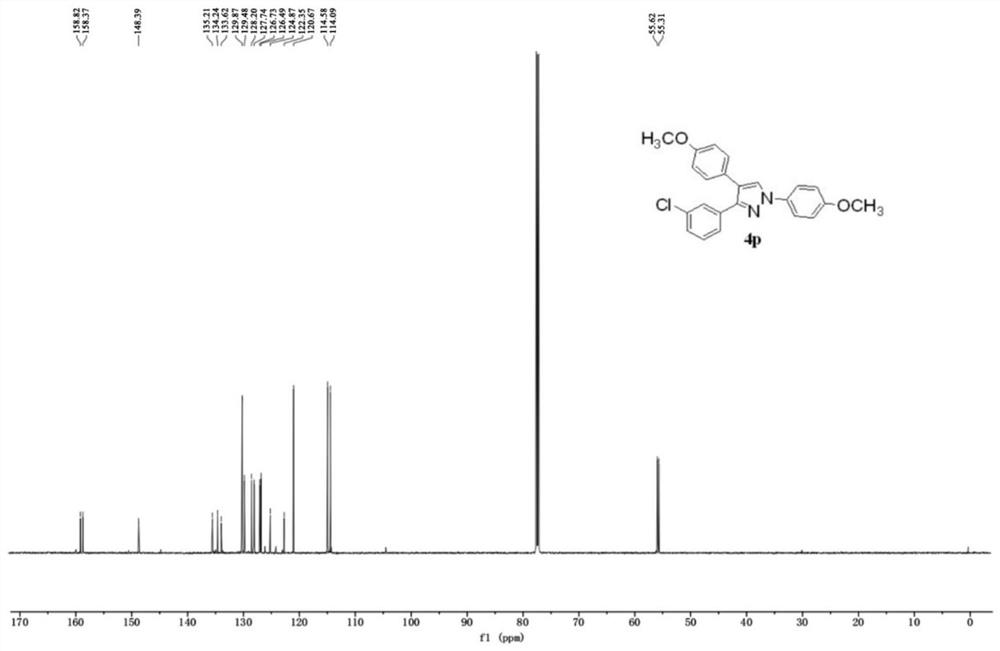
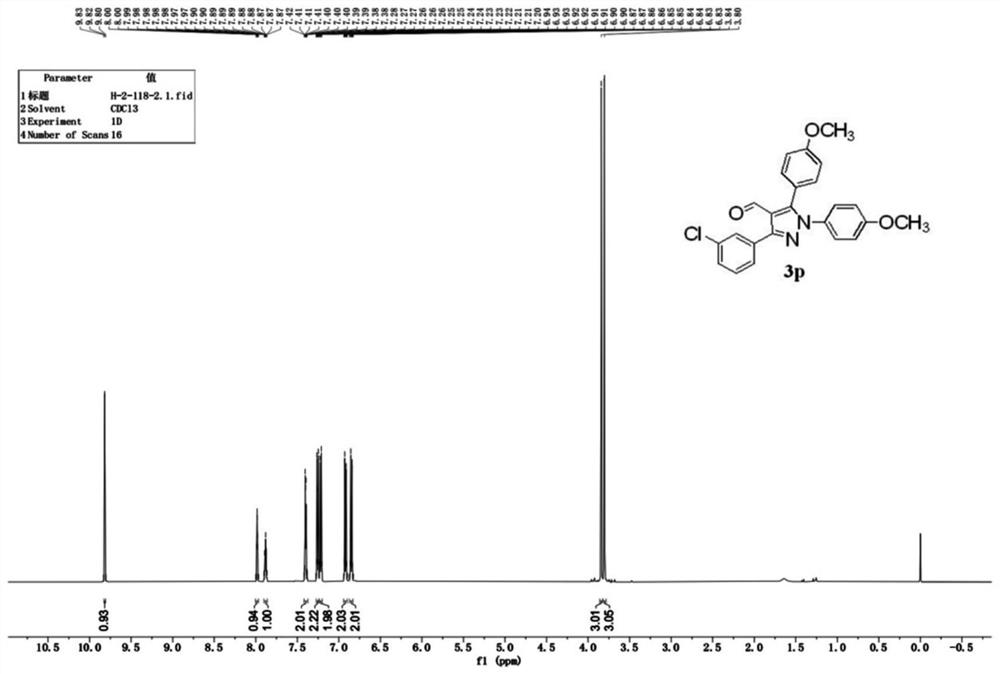
Synthesis method of polysubstituted pyrazole compound
A synthesis method and compound technology, applied in the field of synthesis of multi-substituted pyrazole compounds, can solve the problems of unfriendly environment, uneconomical atoms, cumbersome operation, etc., achieve non-toxic and harmless solvents, increase the scope of application, and simple reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In this example, the compound 1,3,4-triphenyl-1H-pyrazole of the formula III-1 was prepared by using the compound N-phenylbenzohydrazone chloride of the formula I-1 and the compound cinnamaldehyde of the formula II-1 as standard substrates And formula IV-1 compound 1,3,5-triphenyl-1H-pyrazole-4-formaldehyde, its reaction formula is:
[0036]
[0037] The specific operation is: pre-dry the 10mL reaction tube and the magnetic stir bar, and then add 100mg N-phenylbenzohydrazone acid chloride (0.435mmol) and 150.2mg K 2 CO 3 (1.087mmol), then add 2.2mL ethanol solvent, finally add 69.00mg cinnamaldehyde (0.522mmol), the whole reaction is placed in normal temperature to react, TLC monitors the whole reaction process, after the reaction finishes, the solvent is concentrated, and the crude product obtained is used A mixture of petroleum ether and ethyl acetate (30 / 1, v / v) was used as the eluent, and 300-400 mesh silica gel was used as the separation resin for column chroma...
Embodiment 2
[0041] In this embodiment, the compound N-phenylbenzohydrazone chloride of formula I-1 and the compound cinnamaldehyde of formula II-1 are used as standard substrates to study the reaction conditions for the synthesis of base-catalyzed multi-substituted pyrazole compounds. The reaction formula for:
[0042]
[0043] Reaction conditions and results are shown in Table 1:
[0044] Table 1
[0045]
[0046] In Table 1, "equiv" means equivalent; temperature "rt" means room temperature; a means that 2.5 equivalents of DDQ have been added to the reaction system; b means anhydrous and oxygen-free conditions.
Embodiment 3
[0048] This embodiment discloses formula III-2 compound (4-bromophenyl)-1,3-diphenyl-1H-pyrazole and formula IV-2 compound 5-(4-bromophenyl)-3-(ring Preparation of hexa-1,3-dien-1-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde:
[0049]
[0050] The 10mL reaction tube and magnetic stirrer were pre-dried, and then 100mg N-phenylbenzohydrazone chloride (0.435mmol) and 150.2mg K 2 CO 3 (1.087mmol), then add 2.2mL ethanol solvent, finally add 110.17mg p-bromocinnamaldehyde (0.522mmol), the whole reaction is placed in normal temperature to react, TLC monitors the whole reaction process, after the reaction finishes, the solvent is concentrated, and the obtained crude The product uses a mixture of petroleum ether and ethyl acetate (30 / 1, v / v) as the eluent, and uses 300-400 mesh silica gel as the separation resin for column chromatography to obtain (4-bromophenyl)-1,3 -Diphenyl-1H-pyrazole (47.4 mg, light yellow oil) and 5-(4-bromophenyl)-3-(cyclohexa-1,3-dien-1-yl)-1-phenyl - 1H-pyra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com