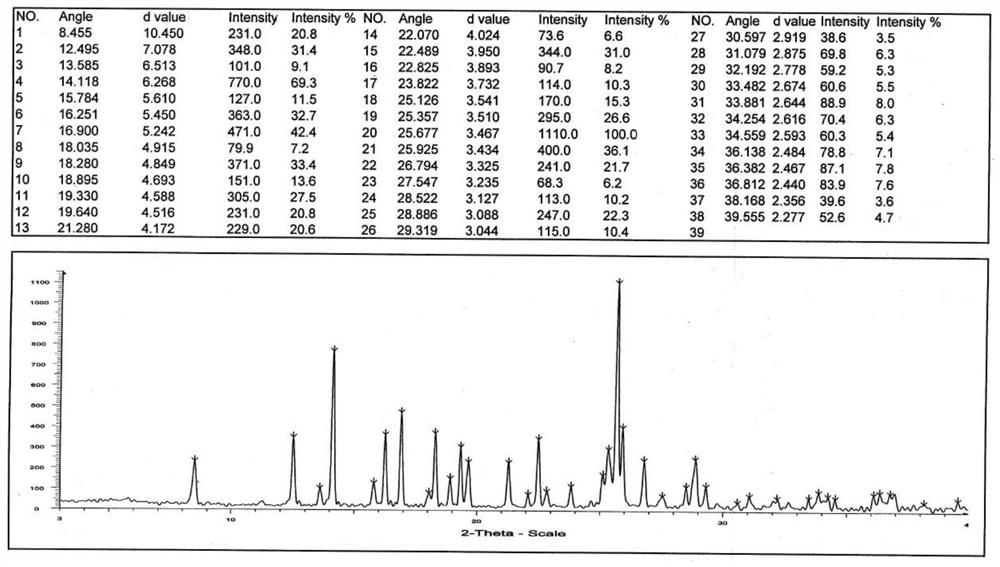
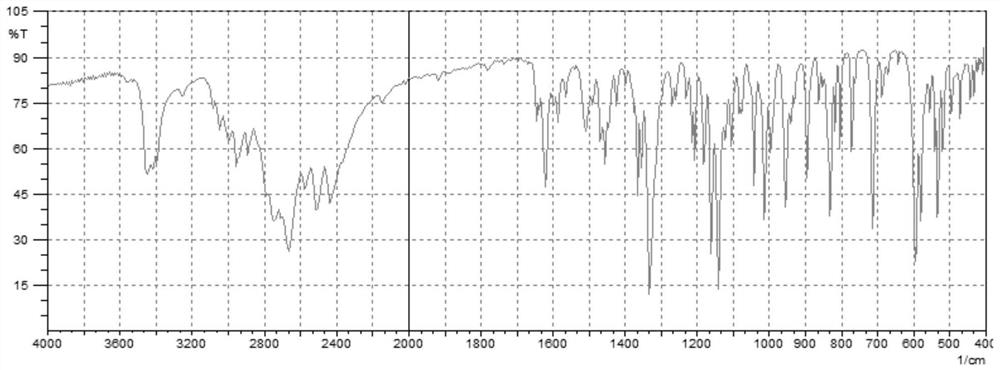
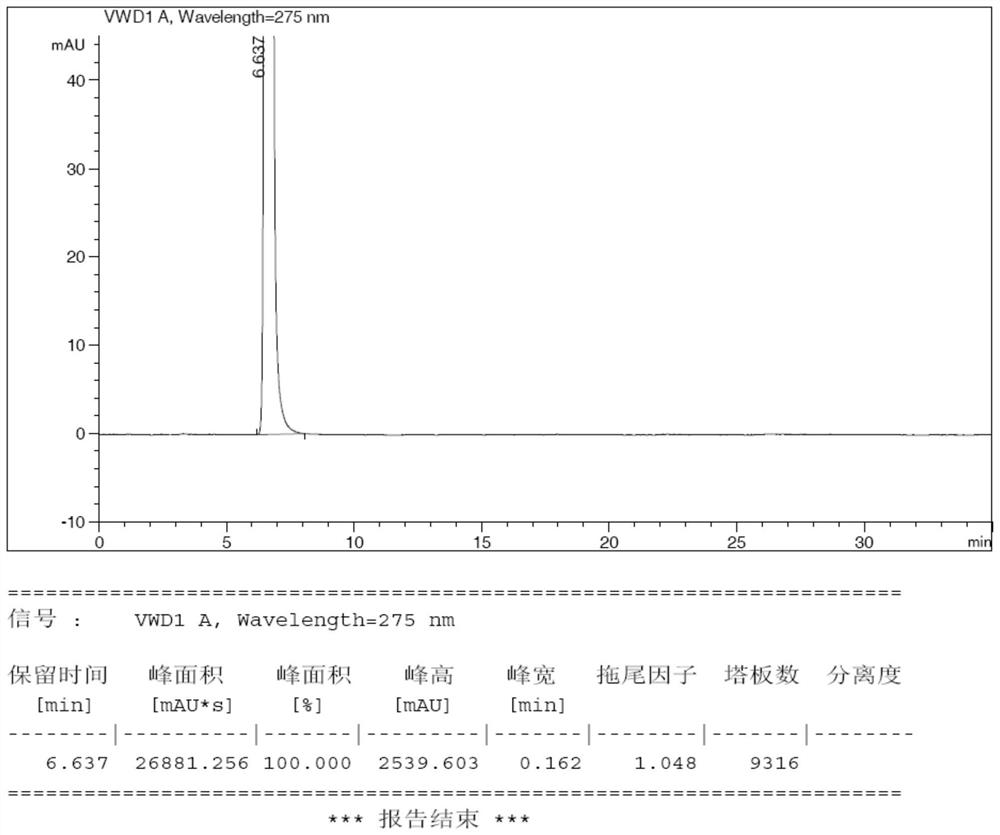
Preparation method of fasudil hydrochloride semihydrate
A technology for fasudil hydrochloride and hemihydrate, applied in the field of medicine, can solve the problems of complicated operation, unavoidable environmental pollution, unfavorable production and the like, and achieve the effect of improving drying efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A preparation method of fasudil hydrochloride hemihydrate, comprising the steps of:
[0049] Referring to Example 3 of US4678783, 55 g of isoquinoline-5-sulfonyl chloride hydrochloride was dissolved in 500 ml of ice water, adjusted to pH 6 with saturated aqueous sodium bicarbonate solution, extracted with 1000 ml of dichloromethane, and the extract was extracted within 20 minutes Add it dropwise to 500ml of dichloromethane solution containing 50g of homopiperazine, cool down in an ice bath, and after the addition is complete, stir the reaction solution at 15-20°C for 2 hours to obtain a fasudil base reaction solution;
[0050] Stir and wash the Fasudil alkali reaction solution with water twice, each water consumption is 1500ml; then take the organic layer, extract with 660ml of 0.35mol / L hydrochloric acid aqueous solution, take the acid extraction water layer; stir and wash the acid with 660ml of dichloromethane Extract the water layer, then use 5mol / L sodium hydroxide ...
Embodiment 2
[0055] A preparation method of fasudil hydrochloride hemihydrate, comprising the steps of:
[0056] With reference to the embodiment 1 of US5942505, 88g homopiperazine is dissolved in 400ml chloroform, cooling in ice bath, the chloroform solution of isoquinoline-5-sulfonyl chloride is added dropwise (50g isoquinoline-5-sulfonyl chloride is dissolved in 400ml chloroform ), added dropwise in 1h, added, and continued to stir in an ice bath for 1h to obtain a fasudil base reaction solution;
[0057] Stir and wash the Fasudil alkali reaction solution with water twice, each water consumption is 800ml; then take the organic layer, extract with 580ml of 0.4mol / L hydrochloric acid aqueous solution, take the acid extraction water layer; stir and wash the acid with 580ml of dichloromethane Extract the water layer, then use 10mol / L sodium hydroxide aqueous solution to adjust the pH of the acid-extracted water layer to 11, cool down to -3~3°C, stir and crystallize for 2h, filter, wash the ...
Embodiment 3
[0060] A preparation method of fasudil hydrochloride hemihydrate, comprising the steps of:
[0061] Referring to Example 2 of CN103450157, in 440ml of dichloromethane, add 7g of potassium carbonate and 25g of homopiperazine, stir to dissolve, control the temperature below 0°C, slowly and continuously add 22g of 5-isoquinolinesulfonyl chloride hydrochloride, and finish adding , stirring at 0-15°C for 3h to obtain a fasudil base reaction solution;
[0062] Stir and wash the Fasudil alkali reaction solution with water twice, each water consumption is 220ml; then take the organic layer, extract with 200ml of 0.45mol / L hydrochloric acid aqueous solution, take the acid extraction water layer; stir and wash the acid with 200ml of dichloromethane Extract the water layer, then use 10mol / L sodium hydroxide aqueous solution to adjust the pH of the acid-extracted water layer to 10, cool down to -3~3°C, stir and crystallize for 2h, filter, wash the filter cake with 40ml of water, and dry i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com