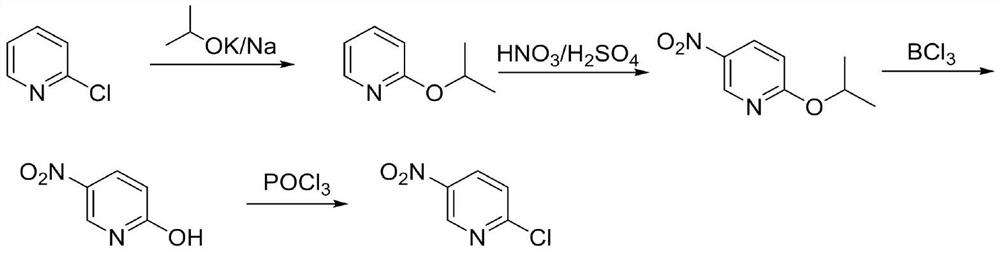
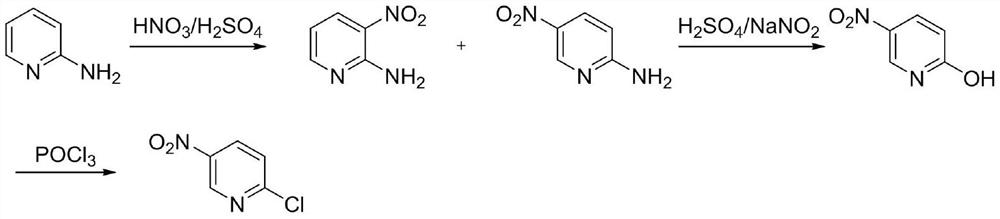
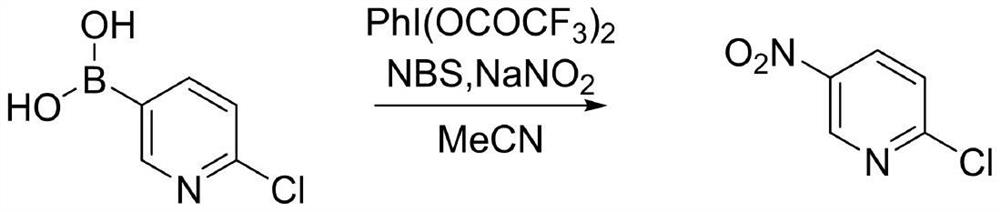
Preparation method of 2-chloro-5-nitropyridine
A technology of nitropyridine and propoxypyridine, which is applied in the field of preparation of 2-chloro-5-nitropyridine, can solve the problems of high price, potential safety hazard, and low yield of direct nitration, and achieves controllable reaction temperature, Improvement of yield and reduction of side reactions of isomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] Under the protection of nitrogen, add 56.8g (0.50mol) of 2-chloropyridine and 400mL of isopropanol to the reaction flask, raise the temperature to 80-85°C, add 54.0g (0.55mol) of potassium isopropoxide in batches, and then raise the temperature to reflux React for 2 hours, evaporate a large amount of isopropanol by concentration under reduced pressure, add 5% acetic acid aqueous solution to adjust pH = 6.4-6.9, continue to concentrate under reduced pressure and evaporate isopropanol, the materials are separated, the water layer is extracted with a small amount of dichloromethane, and combined The organic phase was distilled under reduced pressure at 80-100°C to collect a fraction of 2-isopropoxypyridine 64.1g, yield 93.4%, GC: 99.6%. 1 HNMR (400MHz, CDCl 3 ):8.12-8.10(m,1H),7.51-7.49(m,1H),6.78-6.69(m,2H),5.29-5.26(m,1H),1.35-1.32(m,6H).
Embodiment 2
[0035]
[0036] Under the protection of nitrogen, add 56.8g (0.5mol) 2-chloropyridine and 400mL isopropanol to the reaction flask, raise the temperature to 80-85°C, add 49.2g (0.60mol) sodium isopropoxide in batches, and then raise the temperature to reflux React for 2 hours, evaporate a large amount of isopropanol by concentration under reduced pressure, add 5% acetic acid aqueous solution to adjust pH = 6.4-6.9, continue to concentrate under reduced pressure and evaporate isopropanol, the materials are separated, the water layer is extracted with a small amount of dichloromethane, and combined The organic phase was distilled under reduced pressure at 80-100°C to collect a fraction of 2-isopropoxypyridine 63.6g, yield 92.7%, GC: 99.7%.
Embodiment 3
[0038]
[0039] Under the protection of nitrogen, add 6.86g (0.05mol) 2-isopropoxypyridine and 350mL concentrated sulfuric acid to the reaction flask and mix, slowly add 5.25g (0.06mol) 72% nitric acid dropwise at 20-25°C, and then heat up to 75- React at 80°C for 0.5 hours. After the reddish-brown color appears, control the temperature at 75-85°C, and simultaneously add 54.9g (0.4mol) 2-isopropoxypyridine and 39.4g (0.45mol) 72% concentrated nitric acid dropwise, and react at this temperature for 2 hours . Cool down to room temperature, pour into ice water, extract with MTBE, wash the organic phase with saturated sodium bicarbonate and water, then concentrate under reduced pressure, add n-heptane to beat the temperature, filter to obtain 5-nitro-2-isopropoxypyridine 73.5 g, yield 89.7%, HPLC: 98.9%. 1 HNMR (400MHz, CDCl 3 ):9.25-9.23(m,1H),8.45-8.43(m,1H),6.96-6.94(m,1H),5.37-5.34(m,1H),1.36-1.34(m,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com