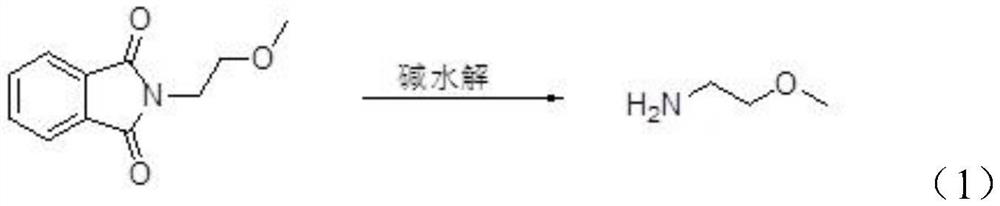
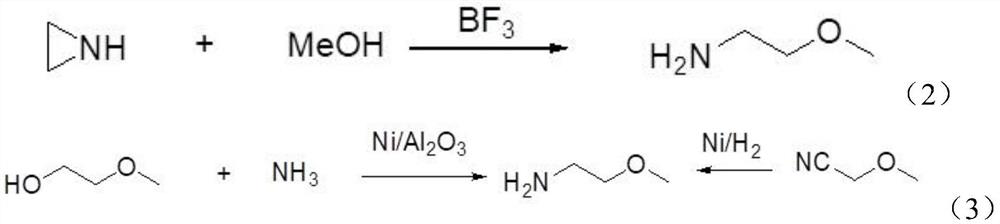
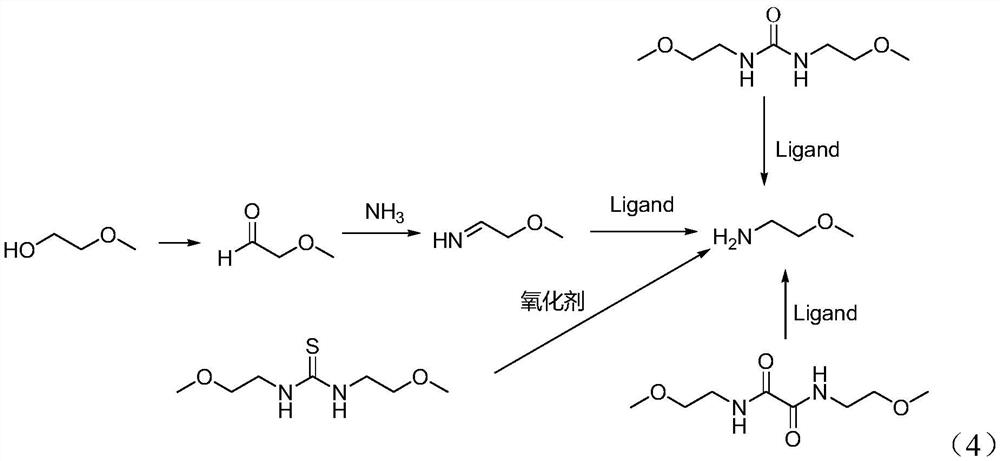
Preparation method of 2-methylethylamine
A technology of methylethylamine and methoxyethylamine, applied in the field of preparation of 2-methylethylamine, can solve the problems of uneconomical atoms, uncontrollable generation of secondary amines and even tertiary amines, etc., and achieves convenient quality. Control, good application prospects, avoid the effect of the use of highly toxic methylating reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The preparation method of 2-methylethylamine according to the embodiment of the present invention comprises the following steps:
[0040] Step S1, using 2-bromoethylamine hydrobromide as a starting material, performing Boc protection on the ethylamine group in 2-bromoethylamine hydrobromide to obtain N-Boc bromoethylamine.
[0041] That is to say, the preparation method of the present invention uses 2-bromoethylamine hydrobromide as a starting material. First, the ethylamine group in the starting material is Boc-protected to obtain intermediate 1, namely N-Boc bromoethylamine.
[0042] The reaction formula is shown in the following formula (8).
[0043]
[0044] Further, in the step S1, the ethylamine group in the 2-bromoethylamine hydrobromide is Boc-protected by Boc anhydride in the first solvent in the presence of a base.
[0045] Furthermore, the first solvent is one or more of dichloromethane, 1,2-dichloroethane, tetrahydrofuran, and ethyl acetate. The starti...
Embodiment 1
[0070] (1) Preparation of N-Boc bromoethylamine
[0071] Add 2-bromoethylamine hydrobromide (800g, 3.9mol) and methylene dichloride in the 10L three-necked flask, control system temperature and drip triethylamine (1261.4g, 9.76mol) successively within room temperature, then control temperature at 10 Below ℃, BOC anhydride (894.8 g, 4.1 mol) was added dropwise, and after the drop was completed, it was naturally raised to room temperature to continue the reaction for 5 hours. Add water to the reaction liquid and stir to separate the liquids. After acid washing with dichloromethane, dry, filter and concentrate, the crude product is pulped with petroleum ether, and 708 g of white solid is obtained from the crude product, which is directly used in the next step without purification.
[0072] (2) Preparation of N-Boc-2-methoxyethylamine
[0073] Add the N-Boc bromoethylamine (700g) and methanol obtained above into a 10L three-necked flask, control the temperature of the system belo...
Embodiment 2
[0083] (1) Preparation of N-Boc bromoethylamine
[0084] Add 2-bromoethylamine hydrobromide (800g, 3.9mol) and dichloromethane in the 10L three-necked flask, control system temperature and drip diisopropylethylamine (987.7g, 9.76mol) successively within room temperature, then control BOC anhydride (894.8 g, 4.1 mol) was added dropwise at a temperature below 10° C. After the drop was completed, it was naturally raised to room temperature to continue the reaction for 5 hours. Add water to the reaction solution and stir to separate the liquids. After acid washing with dichloromethane, dry, filter and concentrate, the crude product is pulped with petroleum ether, and 700 g of white solid is obtained from the crude product, which is directly used in the next step without purification.
[0085] (2) Preparation of N-Boc-2-methoxyethylamine
[0086] Add the N-Boc bromoethylamine (700g) and tetrahydrofuran obtained above into a 10L three-necked flask, control the temperature of the sy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com