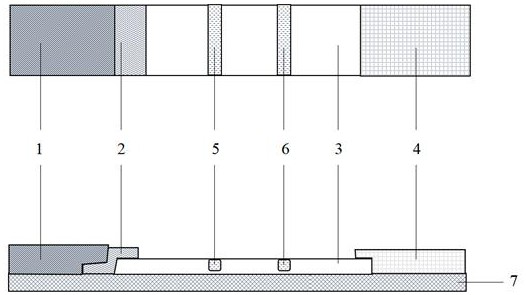
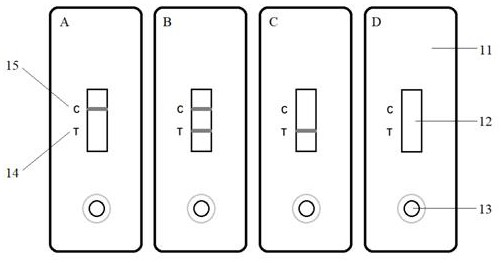
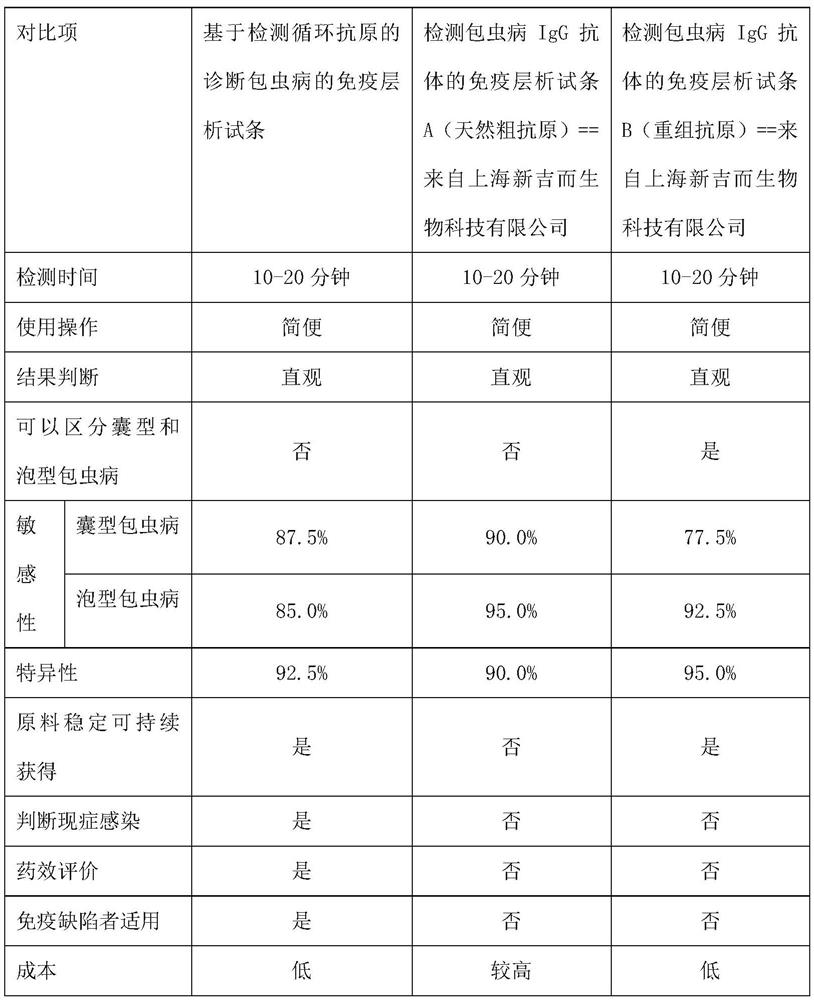
Echinococcosis diagnosis immunochromatography test strip based on circulating antigen detection
An echinococcosis and antigen technology, which is applied in the field of immunochromatographic test strips for the diagnosis of echinococcosis, can solve the problems of not being able to reflect the infection status of echinococcosis, and achieve clear and intuitive results, high sensitivity and specificity, and stability good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of the monoclonal antibody of embodiment 1 echinococcosis cyst fluid antigen
[0033] Preparation of hydatid disease purified cyst fluid antigen: take fresh or frozen sheep liver cyst fluid and centrifuge at 3000rpm for 30 minutes to remove the precipitate. The supernatant was dialyzed overnight against 5 mM acetate buffer solution with pH 5.0, and the dialysate was centrifuged at 5000 rpm for 30 minutes to collect the precipitate. Dissolve the precipitate with 200mM PBS at pH 8.0, then add saturated ammonium sulfate to 40%, let stand at 4°C for 12 hours; centrifuge at 13000rpm for 30 minutes to remove the precipitate, and dialyze the supernatant against pH 7.4, 10mM PBS overnight. The dialysate was recovered and the protein concentration was measured to obtain the purified cystic fluid antigen of echinococcosis. This antigen is used to prepare monoclonal antibodies.
[0034] Immunization: Each BALB / c mouse was injected intraperitoneally with 100 μg of...
Embodiment 2
[0045] Example 2 Preparation of Immunochromatographic Test Strips for Diagnosis of Echinococcosis Based on Detecting Circulating Antigens
[0046] 1. The monoclonal antibody against hydatid cyst fluid antigen was prepared from Example 1.
[0047] 2. Goat anti-mouse IgG was purchased from Cohesion Biosciences.
[0048] 3. Preparation of Monoclonal Antibody Colloidal Gold Probe Solution
[0049] (1) Colloidal gold is prepared by citric acid reduction method:
[0050] The HAuCl4 (Shanghai Sinopharm Group Chemical Reagent Co., Ltd.) aqueous solution that is 0.01% by mass percent concentration is boiled, and 2 mL of trisodium citrate aqueous solution that is 1% by mass percent concentration is added under stirring, and continues to boil until the liquid is dark red to obtain colloidal gold solution.
[0051] (2) Preparation of immune colloidal gold probe pad:
[0052] with 0.1M K 2 CO 3 Adjust the pH value of the solution to 7.2, add anti-hydatid cyst fluid antigen monoclonal...
Embodiment 3
[0061] Example 3 Laboratory and on-site detection and evaluation of immunochromatographic test strips for the diagnosis of echinococcosis based on detection of circulating antigens
[0062] 1. Detection method
[0063] Sample requirements: The test sample is whole blood or serum, using a blood collection needle to collect peripheral blood (on-site) or using collected venous anticoagulant blood (laboratory). The sample should be tested as soon as possible after collection. If it cannot be tested in time, the collected whole blood sample needs to wait for its natural agglutination to collect serum, which can be stored at 2-8°C for 3 days. If long-term storage is required, it should be stored below -20°C. Avoid repeated freeze-thaw cycles. Samples need to be shipped in vacuum flasks or other devices containing dry ice.
[0064] Detection method: Use a capillary blood collection tube to draw the blood sample to be tested to the scale line (5μL), and put it into the test strip de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com