Mycobacterium tuberculosis fusion protein AR2, construction, expression and purification method and application thereof
A Mycobacterium tuberculosis fusion protein technology, applied in the field of Mycobacterium tuberculosis fusion protein AR2 and its construction, expression and purification, can solve the problems of ineffective prevention of adult tuberculosis and the failure to develop new anti-tuberculosis vaccines, and achieve long-lasting The effects of immune protection, good specificity and biological activity, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
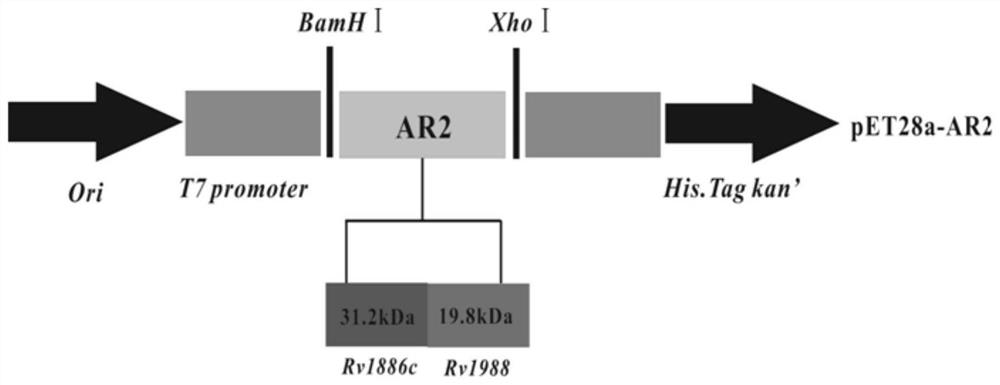
[0090] Example 1: Construction, expression and purification of Mycobacterium tuberculosis fusion protein AR2
[0091] The construction, expression and purification method of mycobacterium tuberculosis fusion protein AR2 comprises the following steps,
[0092] S1: Extract the gene sequences encoded by Rv1988 and Rv1886c from the whole genome sequence of M.tb H37Rv provided by Genbank database;
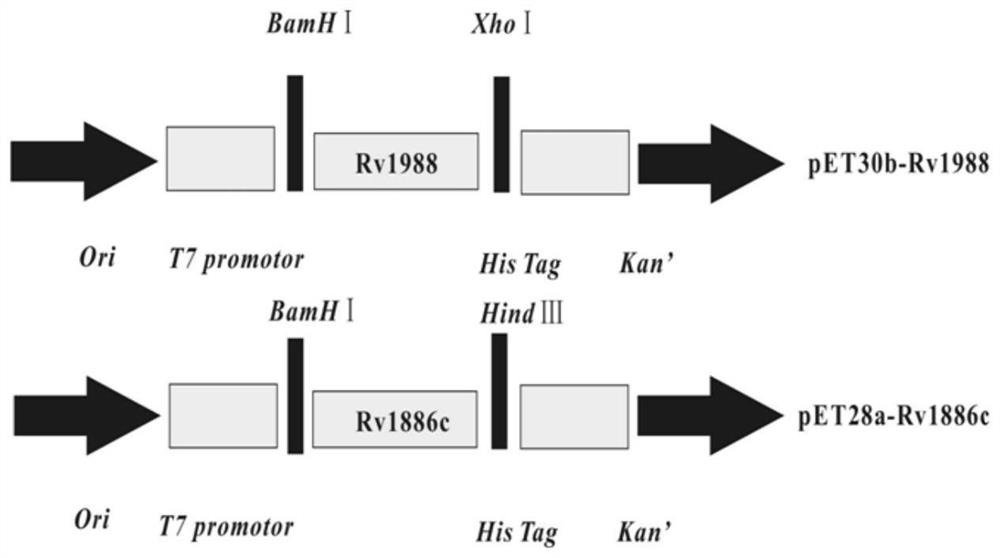
[0093] S2: Using pET28a and pET30b as vectors, construct pET30b-Rv1988 recombinant plasmid and pET28a-Rv1886c recombinant plasmid;
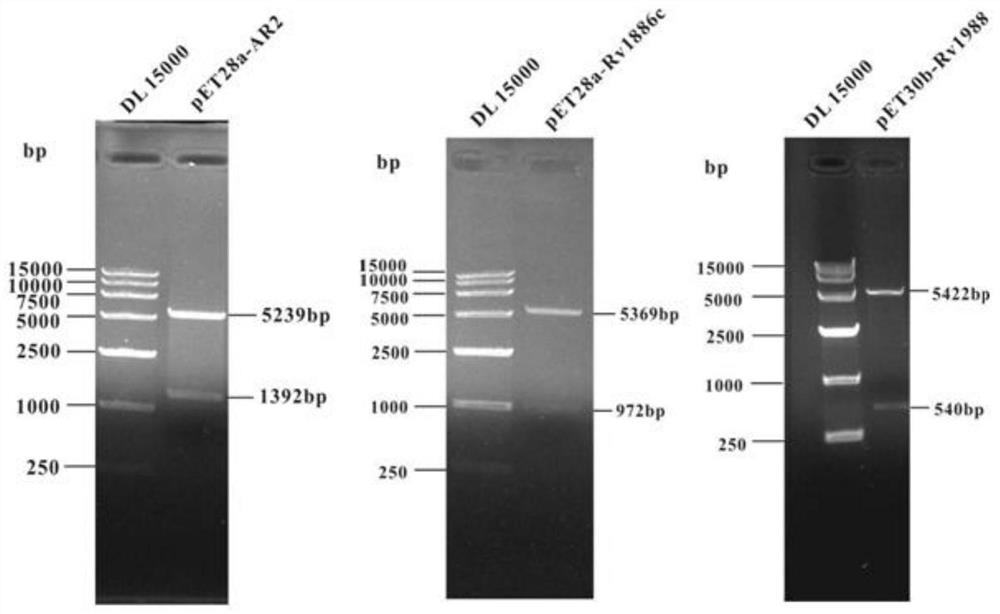
[0094] Specifically, S201: Design primers corresponding to Rv1988 and Rv1886c with reference to the multiple cloning sites of prokaryotic expression vectors pET28a and pET30b, as shown in Table 1 below, and perform PCR gene amplification using the H37Rv genome as a template; reaction conditions for gene amplification and PCR The reaction systems are shown in Table 2 and Table 3 below, respectively.
[0095] Table 1 Gene and primer information
[0096] ...
Embodiment 2
[0244] Embodiment 2 provides the application of Mycobacterium tuberculosis fusion protein AR2 in the preparation of tuberculosis subunit vaccine, the specific application process includes the following steps,
[0245] 1) preparing cationic liposome DDA;
[0246] Cationic liposome DDA was prepared by film method: CHCl 3 and CH 3 OH is mixed as a solvent in a volume ratio of 9:1, and 20 mg of DDA is added and dissolved, and N is slowly introduced 2 Blow dry the above solvents and leave overnight at room temperature to allow them to dry completely. Prepare a 5 mg / ml stock solution with sterilized 1×PBS; bathe in water at 60°C for 1 hour (vortex regularly to fully dissolve it), and cool down.
[0247] 2) using cationic liposome DDA to prepare DC adjuvant, DM adjuvant and DMC adjuvant respectively;
[0248] Specifically, DC adjuvant:
[0249] 5'-T*C*C*A*T*G*A*C*G*T*T*C*C*T*G*A*C*G*T*T-3', oligonucleotide The sequence was synthesized by Suzhou Synbio Biotechnology Co., Ltd., a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com