Olefin hydroformylation reaction method and catalytic system
A technology for olefin hydroformylation and catalytic system, which is applied in the field of olefin hydroformylation reaction and its catalytic system, can solve the problems of easy reduction of catalytic activity, difficult to use directly, difficult to store stably, etc., and achieves the improvement of stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
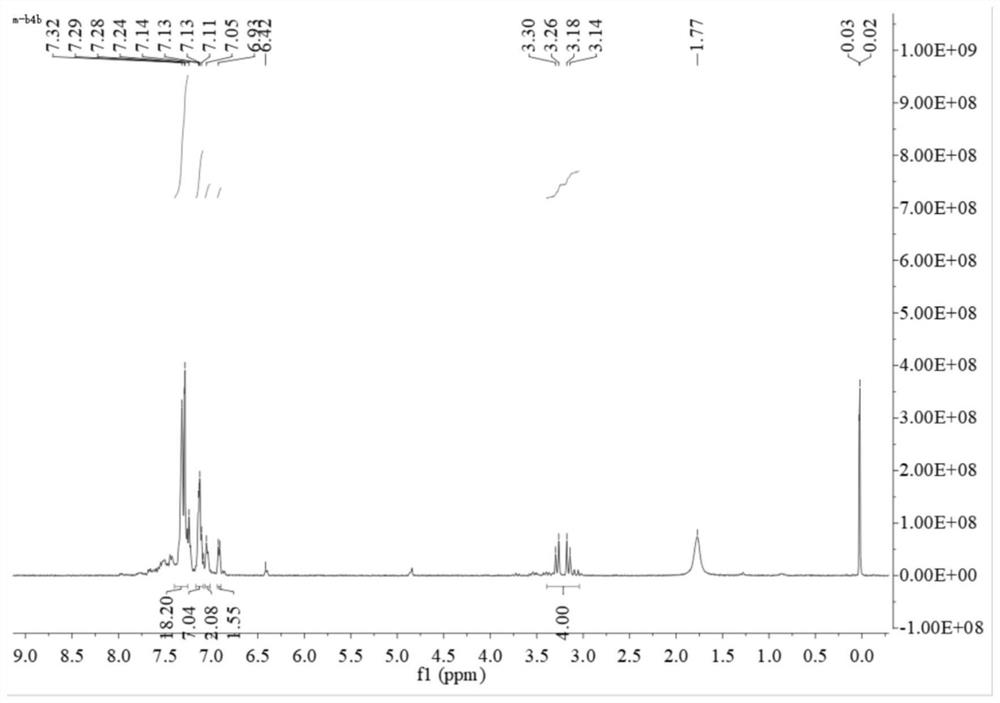
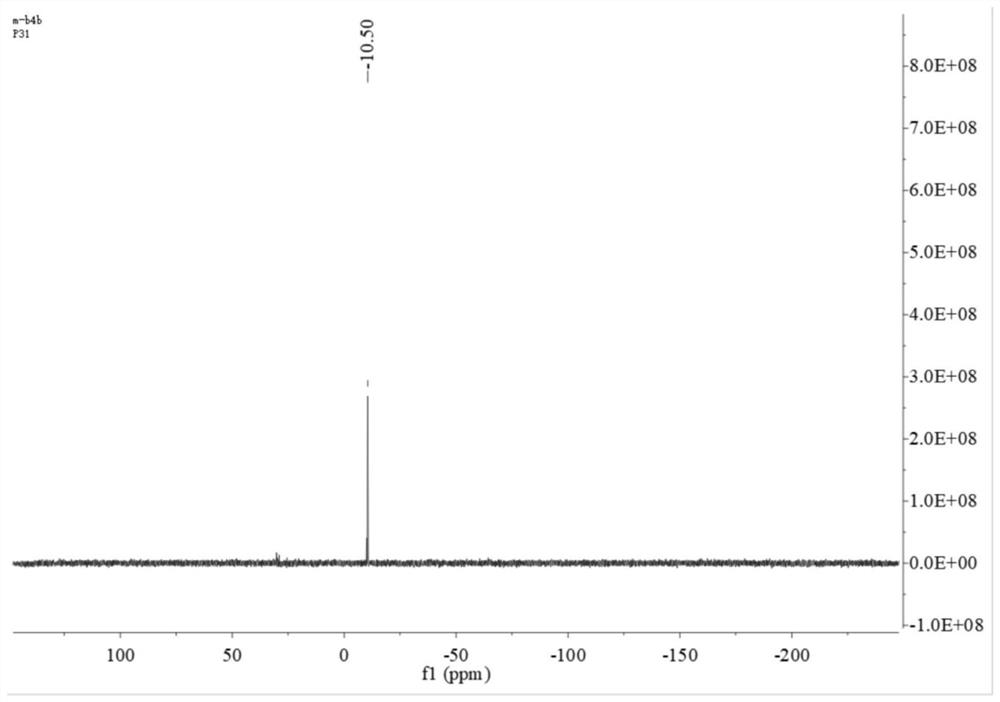
[0046] 1) Synthesis of the bidentate phosphine ligand 2,2'-bis(diphenylphosphinomethylene)-1,1'-biphenyl (BISBI), the reaction formula is as follows:
[0047]
[0048] Specific steps are as follows:
[0049] Weigh 5g of 2,2'-dimethylbiphenyl 1 into 50mL of dichloromethane (DCM), then add 9.3g of N-bromosuccinimide (NBS) and 225mg of azobisisobutyronitrile (AIBN ), reflux reaction under nitrogen protection for 10h. After the reaction, the system was cooled to room temperature, and the precipitated solid was removed by filtration. The filter cake was rinsed once with DCM, the filtrate was collected, washed once with saturated sodium chloride, the organic phase was collected and dried with anhydrous sodium sulfate, and then spin-dried after filtering the desiccant. The solvent and the residue were recrystallized with n-hexane. After crystallization, a large amount of white solids precipitated out of the system. The white solids were collected by suction filtration and dried to ...
Embodiment 2
[0058] The synthesis of the bidentate phosphine ligand BISBI, the preparation of the catalytic system 2 and its application in the hydroformylation of 1-butene are basically the same as in Example 1, except that the paraffin wax is replaced by palm wax.
[0059] The catalytic activity data of catalytic system 2 after being stored in air at room temperature for 30 days is as follows: the conversion rate of 1-butene is 92.1%, and the ratio of n-valeraldehyde and 2-methylbutyraldehyde in the product is 18:1.
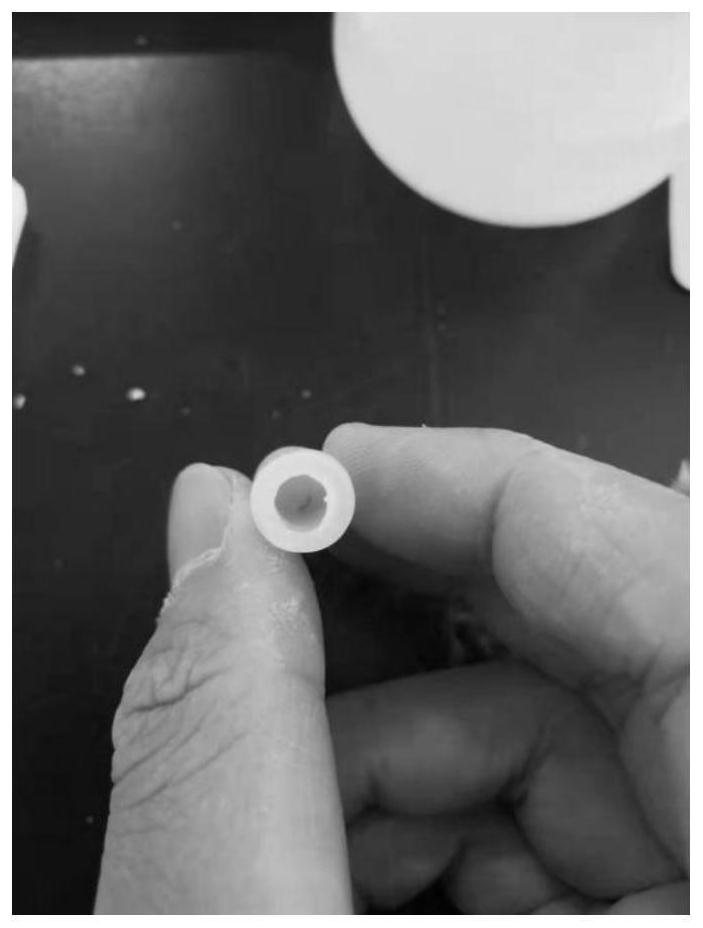
[0060] After the catalytic system 2 was placed in water for 30 days, the capsule body did not break. It was also applied to the hydroformylation reaction of olefins. It was found that the catalytic activity data was as follows: the conversion rate of 1-butene was 91.7%, and the n-valeraldehyde and 2-butene in the product were - The ratio of methylbutyraldehyde is 18:1.
Embodiment 3
[0062] The synthesis of the bidentate phosphine ligand BISBI, the preparation of the catalytic system 3 and its application in the hydroformylation of 1-butene are basically the same as in Example 1, the only difference being that the metal-based catalyst is prepared from 0.05 g of tri(triphenyl Phosphine) rhodiumcarbonyl hydride was replaced by 0.5 g cobalt carbonyl.
[0063] The catalytic activity data of catalytic system 3 after being stored in air at room temperature for 20 days are as follows: the conversion rate of raw materials is 47%, and the molar ratio of n-valeraldehyde and 2-methylbutyraldehyde is 15:1.
[0064] After the catalytic system 3 was placed in water for 20 days, the capsule body did not break. It was also applied to the hydroformylation reaction of olefins. It was found that the catalytic activity data were as follows: the conversion rate of raw materials was 46%, n-valeraldehyde and 2-methylbutyraldehyde The molar ratio is 15:1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com