Method for synthesizing poly (1, 4-butanediol succinate) through ring-opening polymerization
A technology of polysuccinate and butylene glycol ester, which is applied in the field of ring-opening polymerization to synthesize polybutanediol succinate, can solve the problems of intermittent operation, high production cost, and poor environmental protection, and achieve Improve efficiency and production capacity, reduce raw material costs, and improve production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
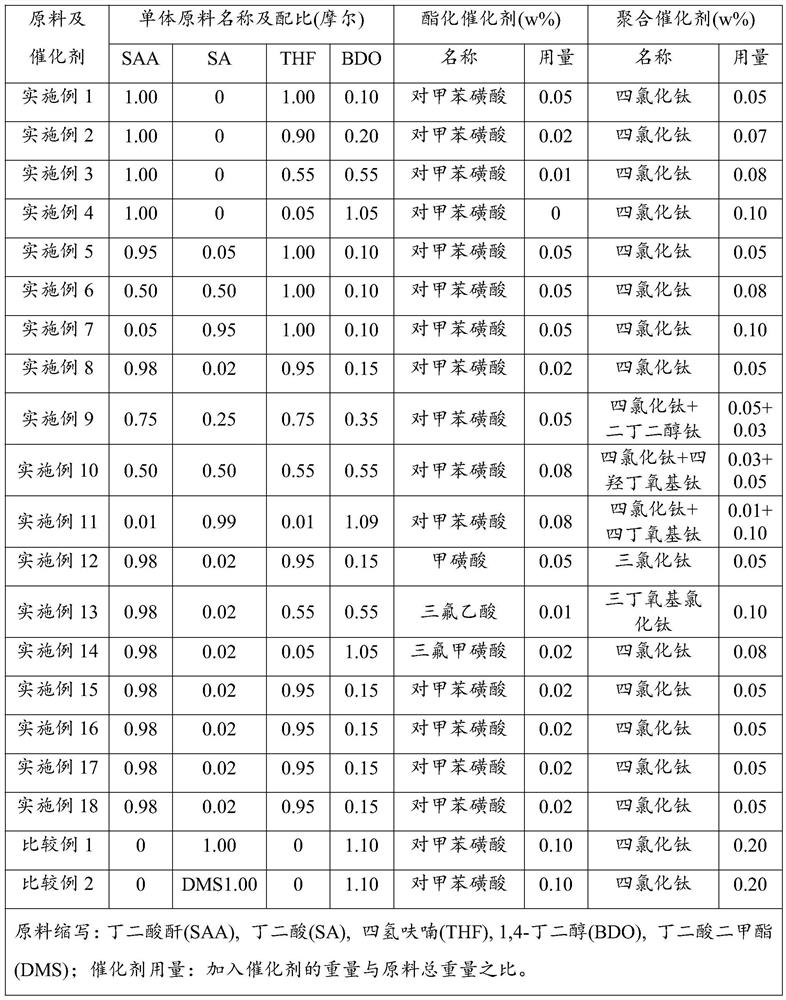
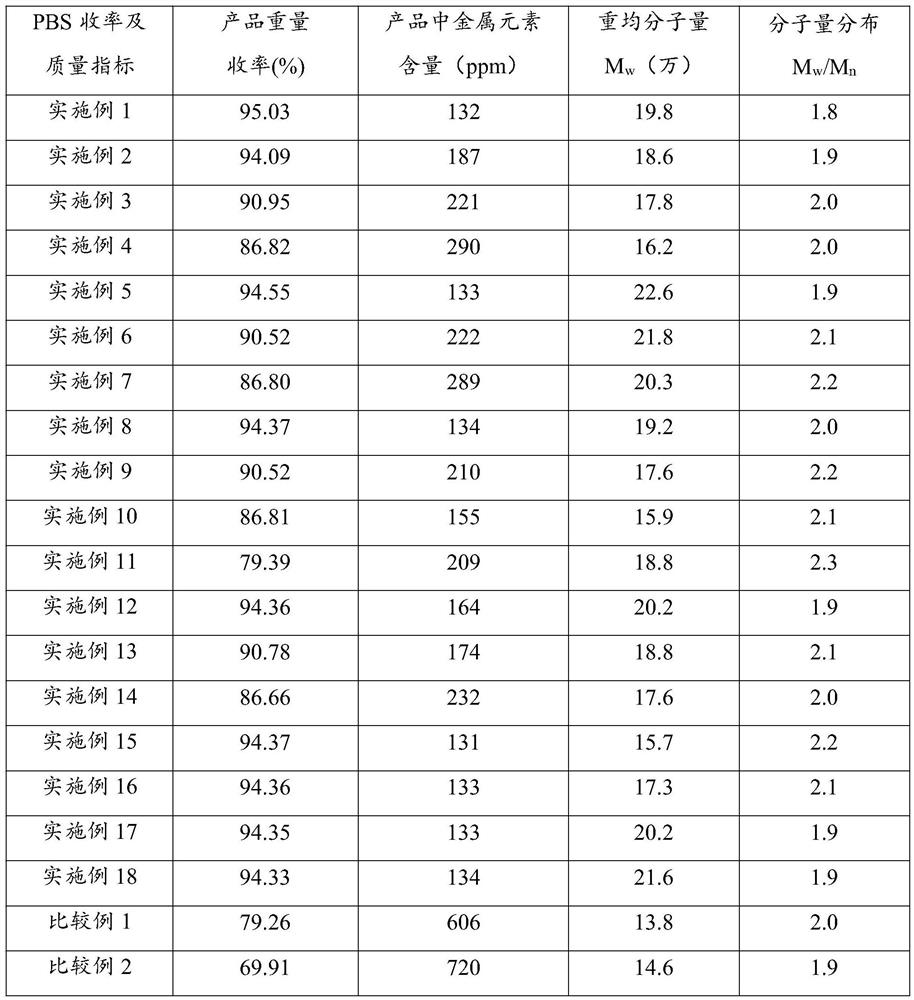
[0032] Without adding succinic acid and changing the ratio of tetrahydrofuran and 1,4-butanediol, succinic anhydride, tetrahydrofuran and 1,4-butanediol were synthesized by ring-opening polymerization to synthesize PBS. The specific synthesis process and result analysis are as follows:
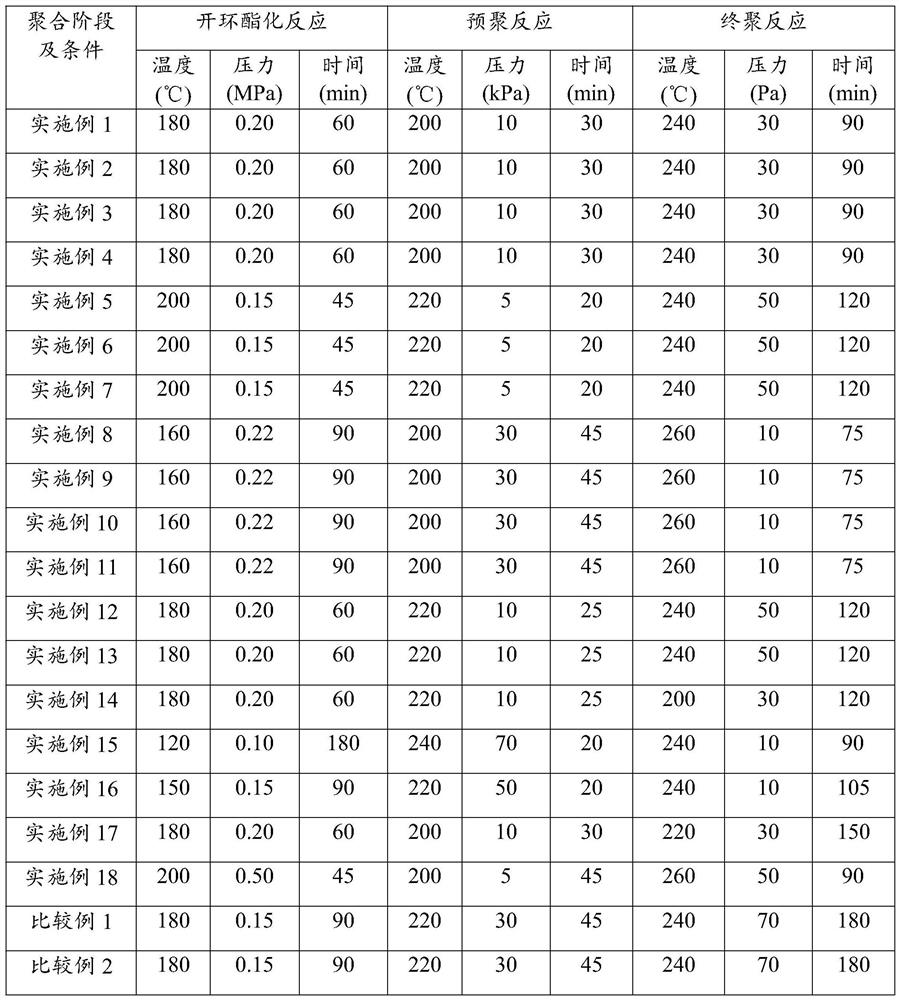
[0033] Add the cyclic monomer succinic anhydride, the cyclic monomer tetrahydrofuran and the chain monomer 1,4-butanediol into the raw material mixing tank according to the metering ratio in Table 1, stir well and preheat to 50°C, then Continuously feed into the esterification reactor and mix uniformly with all p-toluenesulfonic acid and 50wt% titanium tetrachloride (see Table 1 for the amount), and carry out the ring-opening esterification reaction at a temperature of 180°C, a pressure of 0.2MPa and a residence time of 60min ; The esterification product is continuously sent into the decompression kettle, and after the small molecules in the esterification product are decompressed at a pressure...
Embodiment 5~7
[0036] In the case of adding succinic acid and fixing the ratio of tetrahydrofuran and 1,4-butanediol, the ring-opening polymerization of succinic anhydride, tetrahydrofuran and 1,4-butanediol is used to synthesize PBS. The specific synthesis process and results are analyzed as follows:
[0037] Add the cyclic monomer succinic anhydride, the chain monomer succinic acid, the cyclic monomer tetrahydrofuran and the chain monomer 1,4-butanediol according to the metering ratio in Table 1, respectively, into the raw material mixing tank, and stir evenly and preheated to 45°C, and then continuously sent to the esterification reactor and mixed with all the p-toluenesulfonic acid and all the titanium tetrachloride (see Table 1 for the amount), and carried out at a temperature of 200°C, a pressure of 0.15MPa and a residence time of 45min. Ring-opening esterification reaction; then the esterification product is continuously sent into the vacuum kettle, and after the small molecules in the...
Embodiment 8~11
[0040] In the case of changing the ratio of four monomer raw materials and the type of polymerization catalyst, succinic anhydride, succinic acid, tetrahydrofuran and 1,4-butanediol were synthesized by ring-opening polymerization. The specific synthesis process and results are analyzed as follows:
[0041] Add the cyclic monomer succinic anhydride, the chain monomer succinic acid, the cyclic monomer tetrahydrofuran and the chain monomer 1,4-butanediol according to the metering ratio in Table 1, respectively, into the raw material mixing tank, and stir evenly And preheated to 60°C, then continuously sent into the esterification reactor and all the p-toluenesulfonic acid, the dosage is shown in Table 1) and mixed evenly, and the ring-opening esterification reaction was carried out at a temperature of 160°C, a pressure of 0.22MPa and a residence time of 90min; Then the esterification product is continuously sent into the decompression kettle, and after the small molecules in the e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com