Pharmaceutical composition containing sitagliptin and metformin, and preparation method thereof
A technology for metformin and a composition, which is applied in the field of pharmaceutical compositions containing sitagliptin and metformin and the preparation thereof, can solve the problems of increased substance content, decreased stability and the like, and can reduce the generation of related substances, reduce content, The effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
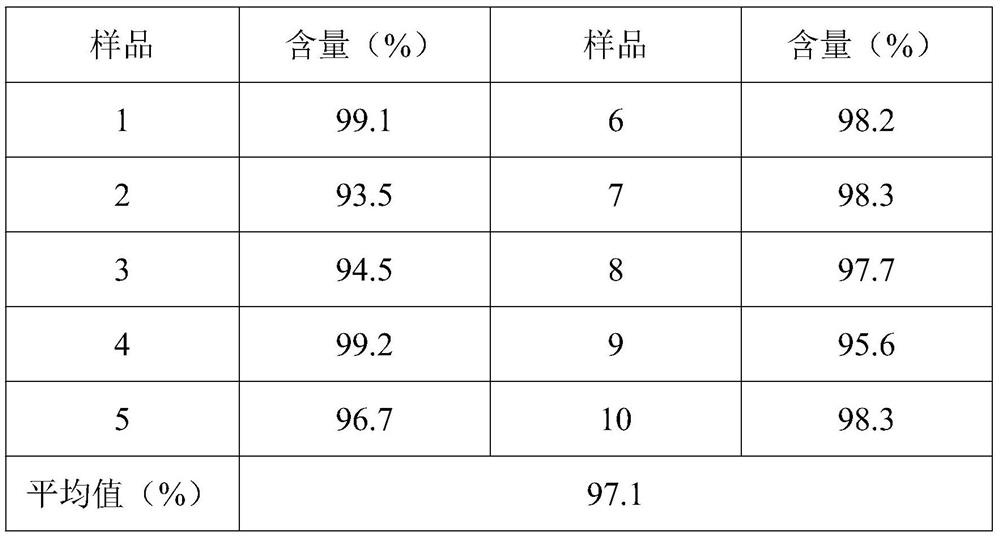
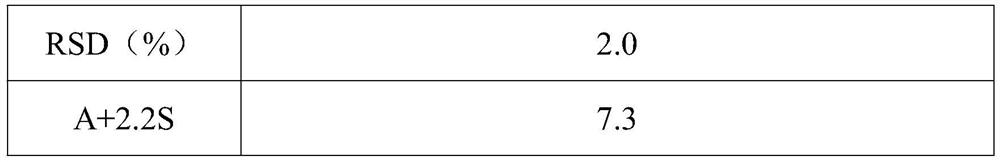
[0027] A pharmaceutical composition containing sitagliptin and metformin, consisting of a metformin sustained-release layer and a sitagliptin immediate-release layer; the metformin sustained-release layer is composed of the following raw and auxiliary materials in parts by weight: 200 parts of metformin hydrochloride; 55 parts of methylcellulose; 9 parts of polyvinylpyrrolidone; 36 parts of pure water; 3 parts of magnesium stearate; 100 parts of lactose monohydrate; 1.5 parts of hypromellose; 20 parts of pure water; 0.75 parts of magnesium stearate; 0.38 parts of L-ascorbic acid.
[0028] Its preparation method comprises the following steps:
[0029] (1) Preparation of metformin sustained-release mixed granules:
[0030] After mixing 200 parts of metformin hydrochloride and 55 parts of hypromellose in a high-efficiency wet granulator, spray 36 parts of pure water, 9 parts of polyvinylpyrrolidone, 1.5 parts of L- Ascorbic acid mixed with the binder mixture, the soft material ...
Embodiment 2
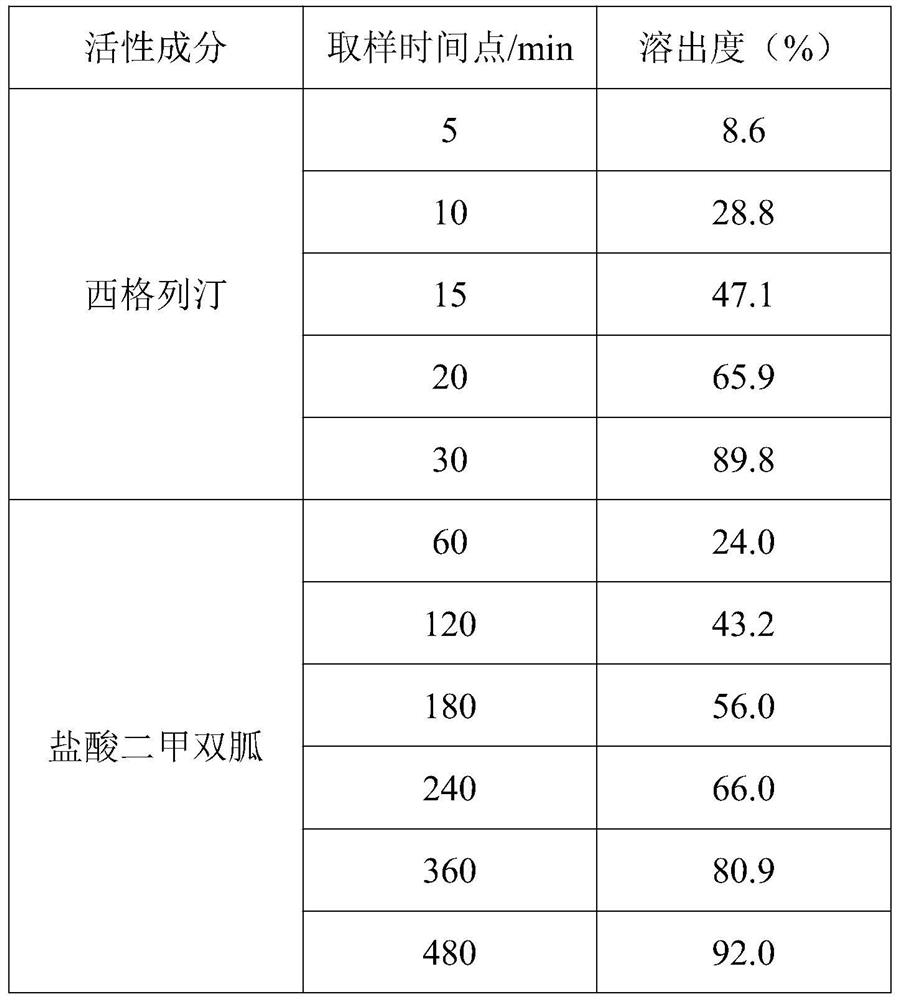
[0036] A pharmaceutical composition containing sitagliptin and metformin, consisting of a metformin slow-release layer and a sitagliptin immediate-release layer; the composition of the metformin sustained-release layer is the same as in Example 1; the sitagliptin immediate release The layer comprises the following raw and auxiliary materials in parts by weight: 50 parts of sitagliptin; 100 parts of mannitol; 1.5 parts of hypromellose; 20 parts of pure water; 0.75 parts of magnesium stearate; It is prepared by the same method as in Example 1 according to the formula. After observation, the obtained sample has good appearance, uniform color, and the film coating is intact without damage.
Embodiment 3
[0038] A pharmaceutical composition containing sitagliptin and metformin, consisting of a metformin sustained-release layer and a sitagliptin immediate-release layer; the metformin sustained-release layer is composed of the following raw and auxiliary materials in parts by weight: 300 parts of metformin hydrochloride; 100 parts of methyl cellulose; 20 parts of polyvinyl alcohol; 50 parts of pure water; 2.5 parts of calcium stearate; 60 parts of gliptin; 80 parts of mannitol; 3 parts of polyvinyl alcohol; 15 parts of pure water; 0.8 parts of calcium stearate; 0.8 parts of propyl gallate.
[0039] Its preparation method comprises the following steps:
[0040] (1) Preparation of metformin sustained-release mixed granules:
[0041] After mixing 300 parts of metformin hydrochloride and 100 parts of hypromellose in a high-efficiency wet granulator, spray 50 parts of pure water, 20 parts of polyvinyl alcohol, and 3 parts of gallic acid with a high-pressure atomizing two-fluid spray gu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com