Preparation method of idosaban and intermediate thereof
A technology of reaction time and methylation, applied in the direction of organic chemistry, can solve the problems of difficult large-scale production, troublesome post-processing, low yield, etc., and achieve the effect of short reaction steps, simple post-processing, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
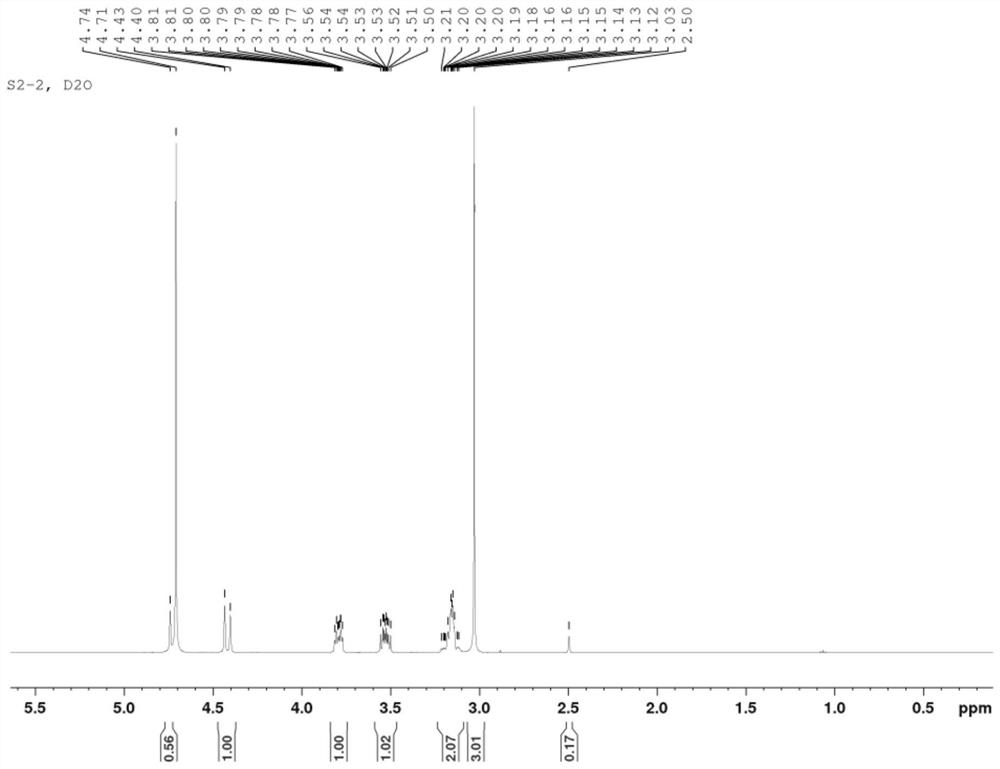
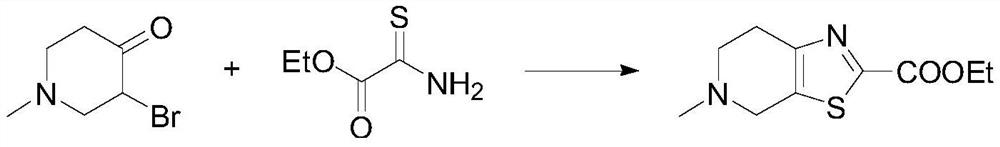
[0046] Dissolve 3-bromo-1-methyl-piperidin-4-one (10mmol, 1.92g) and ethyl thiooxamide (11mmol, 1.46g) in 10mL of ethanol, add sodium carbonate (2mmol, 212mg), Reaction under reflux conditions for 10h. After the reaction, cool to room temperature, filter the insoluble matter, evaporate the filtrate to dryness, add aqueous sodium bicarbonate solution to wash until slightly alkaline, then extract with ethyl acetate, dry, concentrate, and separate through column to obtain 5-methyl-4 , Ethyl 5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxylate.
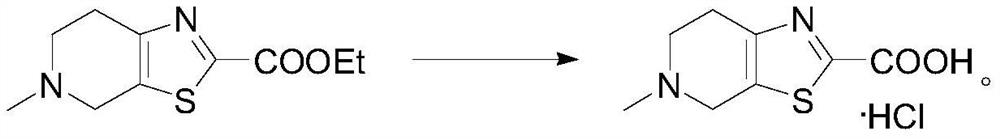
[0047] It was further dissolved in 10 mL of ethanol, 4 mL (0.02 mol) of 5 mol / L NaOH aqueous solution was added, and the reaction was refluxed for 4 h. After cooling to room temperature, distill off the solvent, dissolve the residue with water, extract with ethyl acetate / water to remove organic impurities, adjust the pH of the aqueous phase to 2 with concentrated hydrochloric acid (37%) in an ice bath, and stir to precipitate a white so...
Embodiment 2
[0049] 3-Bromo-1-methyl-piperidin-4-one (10mmol, 1.92g) and ethyl thiooxamide (11mmol, 1.46g) were dissolved in 10mL ethanol, NaOH (2mmol, 80mg) was added, and Reaction under reflux conditions for 10h. After the reaction, cool to room temperature, filter the insoluble matter, evaporate the filtrate to dryness, add aqueous sodium bicarbonate solution to wash until slightly alkaline, then extract with ethyl acetate, dry, concentrate, and separate through column to obtain 5-methyl-4 , Ethyl 5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxylate.
[0050] It was further dissolved in 10 mL of ethanol, 4 mL (0.02 mol) of 5 mol / L NaOH aqueous solution was added, and the reaction was refluxed for 4 h. After cooling to room temperature, distill off the solvent, dissolve the residue with water, extract with ethyl acetate / water to remove organic impurities, adjust the pH of the aqueous phase to 2 with concentrated hydrochloric acid (37%) in an ice bath, and stir to precipitate a white so...
Embodiment 3
[0052] Dissolve 3-bromo-1-methyl-piperidin-4-one (10mmol, 1.92g) and ethyl thiooxamide (11mmol, 1.46g) in 10mL of methanol, add sodium carbonate (2mmol, 212mg), Reaction under reflux conditions for 16h. After the reaction, cool to room temperature, filter the insoluble matter, evaporate the filtrate to dryness, add aqueous sodium bicarbonate solution to wash until slightly alkaline, then extract with ethyl acetate, dry, concentrate, and separate through column to obtain 5-methyl-4 , Ethyl 5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxylate.
[0053] It was further dissolved in 10 mL of ethanol, 4 mL (0.02 mol) of 5 mol / L NaOH aqueous solution was added, and the reaction was refluxed for 4 h. After cooling to room temperature, distill off the solvent, dissolve the residue with water, extract with ethyl acetate / water to remove organic impurities, adjust the pH of the aqueous phase to 2 with concentrated hydrochloric acid (37%) in an ice bath, and stir to precipitate a white s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com