Method for synthesizing citicoline sodium
A technology for synthesizing citicoline and citicoline sodium, which is applied in the fields of chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of inability to carry out large-scale industrial production, poor atom economy, reaction Solve problems such as cumbersome operation, achieve the effect of novel design route, reduce loss, and shorten reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
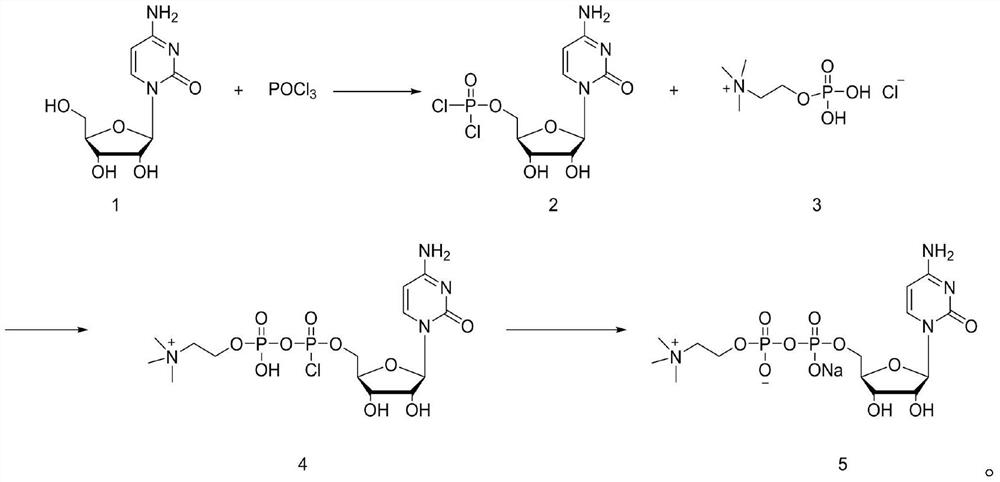
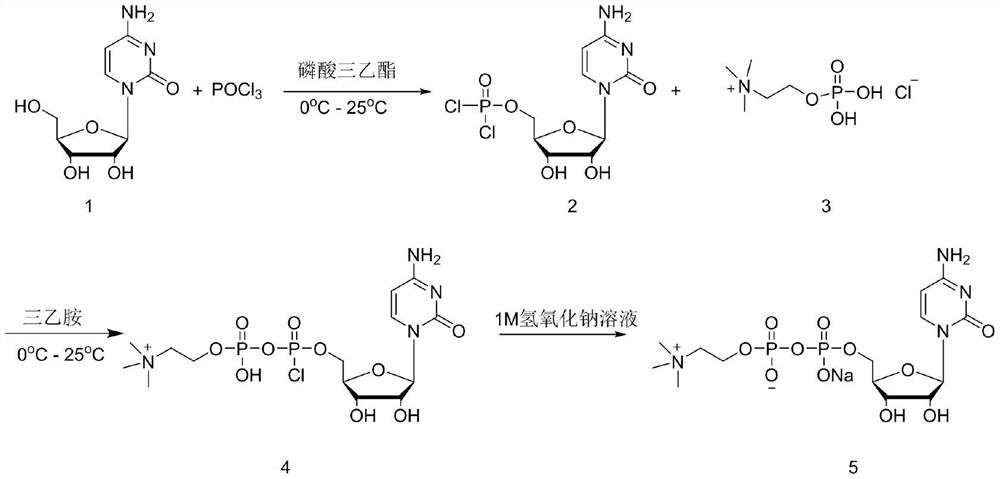
[0029] In the first step, 20g (0.082mol) of cytidine was weighed and dispersed in 100mL of triethyl phosphate, the temperature was lowered to 0°C, and 13.8g (0.09mol) of phosphorus oxychloride was slowly added. Warm up to room temperature and stir for 12 hours, follow the reaction in the liquid phase until the raw material cytidine basically disappears.
[0030] In the second step, the reaction solution was lowered to 0°C, and 20.2g (0.2mol) of triethylamine was added. After stirring evenly, phosphorylcholine chloride was added in batches. After the addition, the temperature was raised to 25°C for 24 hours, and then the reaction The solution was lowered to 0°C, and 80mL of 1M sodium hydroxide aqueous solution was slowly added dropwise. After the dropwise addition, 100mL of water and 100mL of dichloromethane were added to the reaction solution, and the layers were extracted, and the aqueous phase was collected. The organic phase was passed through a rectification tow...
Embodiment 2
[0033]
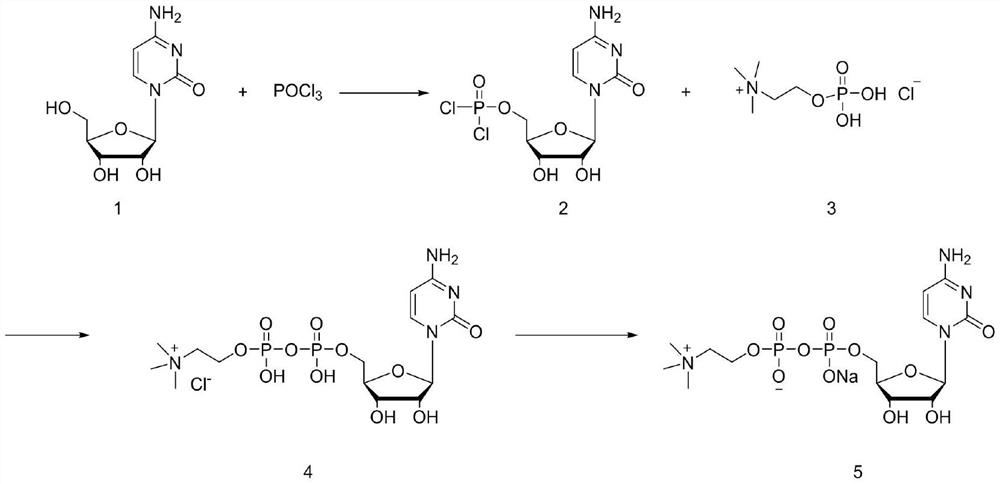
[0034] In the first step, 20g (0.082mol) of cytidine was weighed and dispersed in 100mL of trimethyl phosphate, the temperature was lowered to 0°C, and 13.8g (0.09mol) of phosphorus oxychloride was slowly added. Warm up to room temperature and stir for 12 hours, follow the reaction in the liquid phase until the raw material cytidine basically disappears.
[0035] In the second step, the reaction solution was lowered to 0°C, and 20.2g (0.2mol) of triethylamine was added. After stirring evenly, phosphorylcholine chloride was added in batches. After the addition, the temperature was raised to 25°C for 24 hours, and then the reaction The solution was lowered to 0°C, and 80mL of 1M sodium hydroxide aqueous solution was slowly added dropwise. After the dropwise addition, 100mL of water and 100mL of dichloromethane were added to the reaction solution, and the layers were extracted, and the aqueous phase was collected. The organic phase was passed through a rectification to...
Embodiment 3
[0038]
[0039] In the first step, 20g (0.082mol) of cytidine was weighed and dispersed in 100mL of triethyl phosphate, the temperature was lowered to 0°C, and 15.3g (0.1mol) of phosphorus oxychloride was slowly added. Warm up to room temperature and stir for 12 hours, follow the reaction in the liquid phase until the raw material cytidine basically disappears.
[0040] In the second step, the reaction solution was lowered to 0°C, and 20.2g (0.2mol) of triethylamine was added. After stirring evenly, phosphorylcholine chloride was added in batches. After the addition, the temperature was raised to 25°C for 24 hours, and then the reaction The solution was lowered to 0°C, and 80mL of 1M sodium hydroxide aqueous solution was slowly added dropwise. After the dropwise addition, 100mL of water and 100mL of dichloromethane were added to the reaction solution, and the layers were extracted, and the aqueous phase was collected. The organic phase was passed through a rectification towe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com