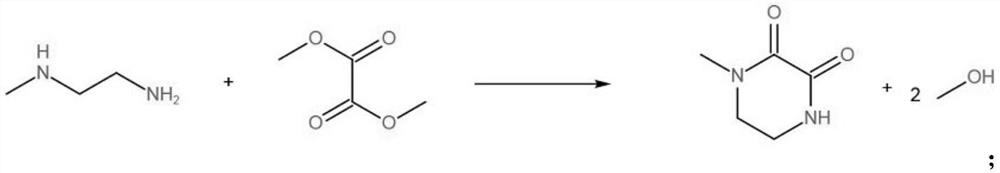
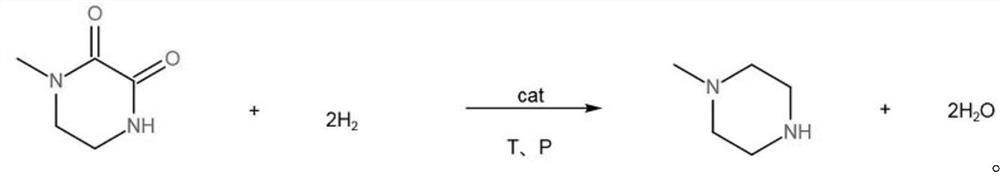
A kind of method for preparing n-methylpiperazine
A technology of methylpiperazine and methylethylenediamine, which is applied in the field of preparing N-methylpiperazine, can solve the problems such as difficulty in obtaining raw materials of diethanolamine, irritation of the respiratory tract, environmental hazards, etc., and achieves high catalytic activity and thermal stability. Stability ability, promotion of reactant conversion, low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Prepare a 1L beaker, add 296.50g (ie 4.0mol) N-methylethylenediamine, 495.97g (ie 4.2mol) dimethyl oxalate, add 50g Raney nickel, transfer the feed liquid into a 1L reaction kettle, After 3 times of nitrogen replacement at room temperature, the reactor was leak tested, filled with hydrogen 0.15MPa for replacement, and heated to 180°C. Continue to charge hydrogen to 4.0MPa and react for 3.0h, control the temperature at 180°C. After the reaction, use circulating water to cool down to 40°C to prevent methanol from volatilizing after the reaction, stop stirring, discharge the hydrogen to the pressure of 0.01MPa, and replace with nitrogen for 3 First, to prevent residual hydrogen in the kettle, open the reactor, recover the reaction solution and catalyst in a beaker, let stand for 1 hour, let the catalyst stand at the bottom of the beaker, and take samples for analysis by gas chromatography.
[0020] The results of gas chromatography analysis showed that the conversion rate ...
Embodiment 2
[0022] Prepare a 1L beaker, add 296.50g (ie 4.0mol) N-methylethylenediamine, 495.97g (ie 4.2mol) dimethyl oxalate, add 50g Raney nickel, transfer the feed liquid into a 1L reaction kettle, After 3 times of nitrogen replacement at room temperature, the reaction kettle was tested for leaks, stirred and mixed evenly, filled with hydrogen at 0.15MPa for replacement, and heated to 150°C. Continue to charge hydrogen to 4.0MPa, react for 3.0h, control the temperature at 150°C, use circulating water to cool down to 40°C, stop stirring, discharge the hydrogen to the pressure of 0.01MPa in the kettle, and replace it with nitrogen for 3 times, then open the reaction kettle and recover the reaction solution. Put the catalyst and the catalyst in a beaker, let stand for 1 hour, let the catalyst stand at the bottom of the beaker, and take a sample for analysis by gas chromatography.
[0023] The results of gas chromatography analysis showed that the conversion rate of N-methylethylenediamine...
Embodiment 3
[0025] Prepare a 1L beaker, add 296.50g (ie 4.0mol) N-methylethylenediamine, 495.97g (ie 4.2mol) dimethyl oxalate, add 50g Raney nickel, transfer the feed liquid into a 1L reaction kettle, After 3 times of nitrogen replacement at room temperature, the reaction kettle was tested for leaks, stirred and mixed evenly, filled with hydrogen 0.15MPa for replacement, and heated to 200°C. Continue to charge hydrogen to 4.0MPa, react for 3.0h, control the temperature at 200°C, use circulating water to cool down to 40°C, stop stirring, discharge the hydrogen to the pressure of 0.01MPa in the kettle, replace with nitrogen three times, open the reactor, and recover the reaction solution Put the catalyst and the catalyst in a beaker, let stand for 1 hour, let the catalyst stand at the bottom of the beaker, and take a sample for analysis by gas chromatography.
[0026] The results of gas chromatography analysis showed that the conversion rate of N-methylethylenediamine was 96.45%, the select...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com