Method for expressing recombinant neurotrophic factor fusion protein, recombinant neurotrophic factor fusion protein and application of recombinant neurotrophic factor fusion protein
A neurotrophic factor and fusion protein technology, applied in the field of functional proteins, can solve the problems of complicated operation, low expression level, and low activity, and achieve the effects of reducing production costs, improving secretion efficiency, and good biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
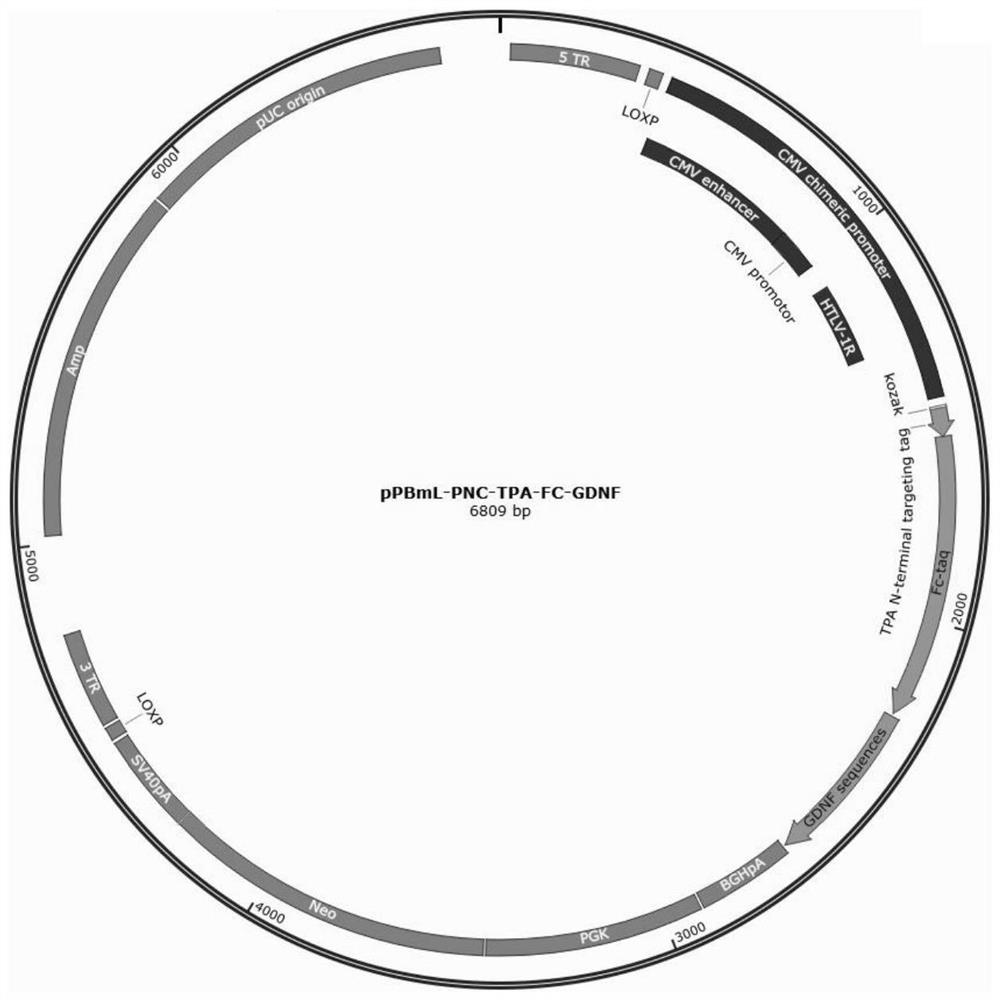
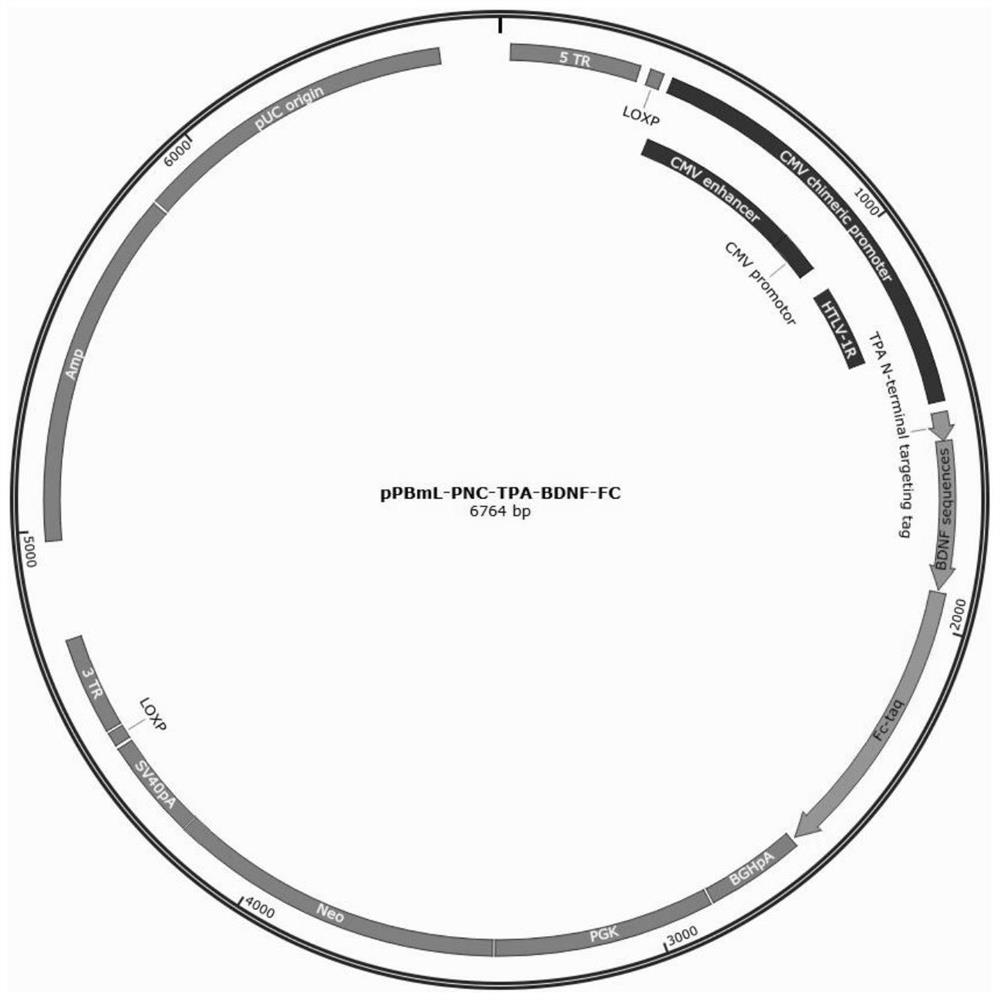
[0077] Construction of high expression plasmids pPBml-PNC-TPA-Fc-GDNF and pPBml-PNC-TPA-BDNF-Fc
[0079] Take the existing pPBml-PNCE plasmid and the artificially synthesized TPA-Fc-GDNF or TPA-BDNF-Fc fusion protein sequence (synthesized by Nanjing GenScript) and digested at 37°C for 2h respectively. The digestion system is shown in Table 5 and Table 5. 6, the endonucleases include Xba I (manufacturer: NEB, product number: R0145L) and BamHI (manufacturer: NEB, product number: R3131L), and Cutsmart is a component in the endonuclease kit.
[0080] Table 5 pPBml-PNCE plasmid digestion system
[0081] component volume XbaI 3μL BamHI 3μL pPBml-PNCE 10μL Cutsmart 3μL ddH 2 O
11μL
[0082] Table 6 fusion protein sequence digestion system
[0083] component volume XbaI 5μL BamHI 5μL TPA-Fc-GDNF or TPA-BDNF-Fc 30μL Cutsmart 5μL ddH 2 O
5μL
...
Embodiment 2
[0091] Construction and screening of high-expressing cell lines
[0092] 1. Electroporation and screening of positive clones
[0093] 1.1 Plasmid electroporation
[0094] Select stable growth HEK293 cells (purchased from Zhuhai Kairui Biotechnology Co., Ltd.), the viability is greater than 95%, take 1 × 10 6 Cells were obtained by centrifugation to remove the supernatant. Resuspend the cells with 100 μL of electroporation buffer 10, add 1 μg pPBml-PNC-TPA-Fc-GDNF or pPBml-PNC-TPA-BDNF-Fc and pEhyPBase plasmids, mix well, and set a blank cell control group (no plasmid), transfer Transfer to the electroporation cup, electroporate with a Lonza electroporator (adjusted to HEK293 ATCC mode), resuspend the cells in DMEM+10% FBS medium after electroporation, spread them in a 6-well plate, and place at 37°C, 5% CO. 2 concentration, and cultivated in a humidified incubator.
[0095] 1.2 Screening of positive clones
[0096] After 24 hours of adherent growth, the adherent HEK293 ce...
Embodiment 3
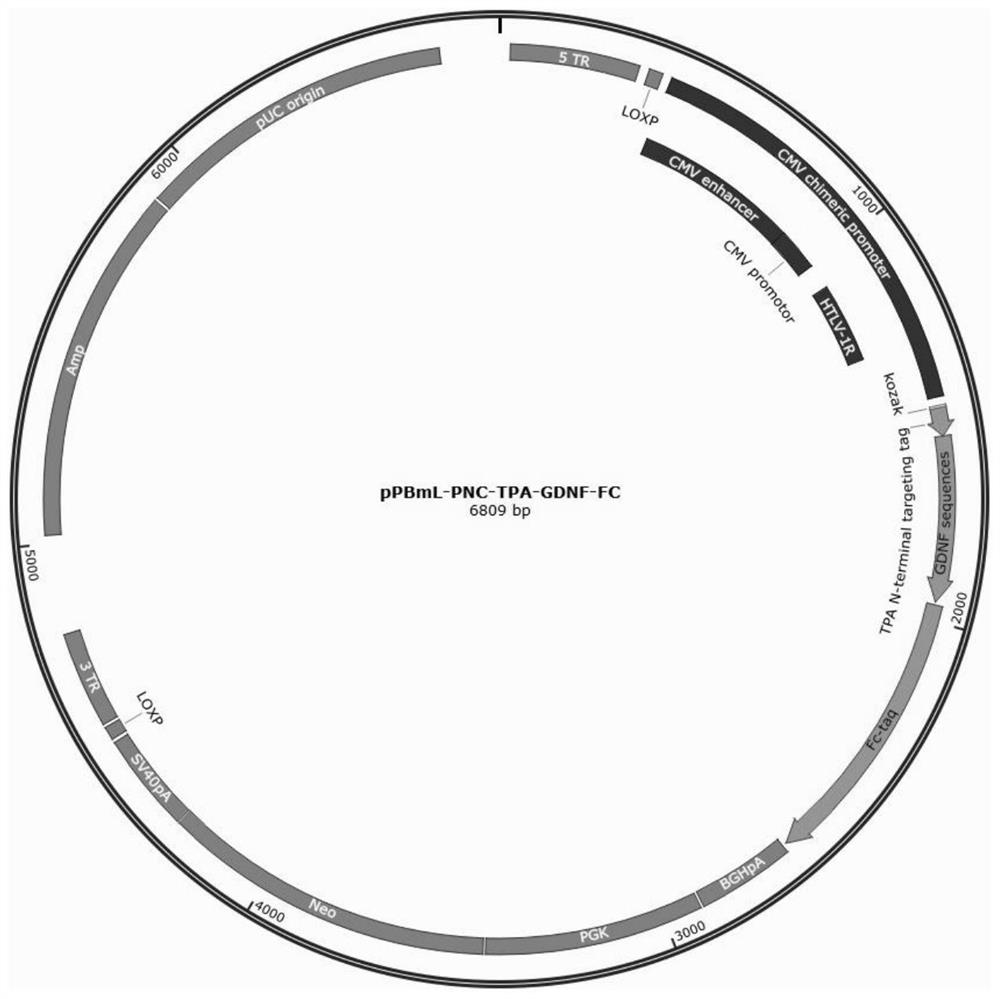
[0098] Recombinant plasmid pPBml-PNC-TPA-GDNF-Fc (for the spectrum, see Figure 1-2 ) and pPBml-PNC-TPA-Fc-BDNF (see Figure 2-2 ) construction method
[0099] According to the method of Example 1, the pPBml-PNCE plasmid and the synthetic TPA-GDNF-Fc or TPA-Fc-BDNF fusion protein sequence were used to construct a recombinant vector. Positive clones were obtained according to the method of Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com