Method for separating and purifying 1, 2, 4-butantriol based on bifunctional ionic liquid
An ionic liquid, separation and purification technology, applied in the separation/purification of hydroxyl compounds, chemical instruments and methods, preparation of hydroxyl compounds, etc., can solve problems such as equipment corrosion, complex extraction methods, and poor industrial practice, and achieve the reduction of aldehydes The effect of low dosage, easy recycling, and long catalytic life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
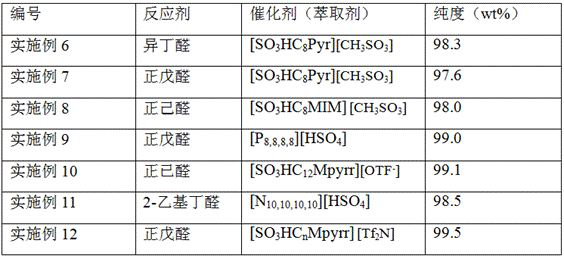
Examples
Embodiment 1
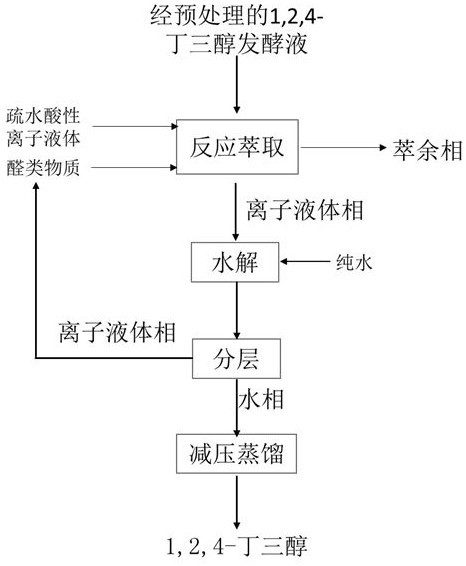
[0030] (1) Take 20ml of pretreated 21g / L 1,2,4-butanetriol fermentation broth and put it in the reactor, add [SO 3 HC 8 MIM][HSO 4 ] ionic liquid 10ml, the reaction temperature is 25 ℃, the reaction extraction time is 1h, the conversion rate of 1,2,4-butanetriol is 95%, 96.5%, 97.2% respectively, the recovery rate of acetal in the ionic liquid phase is respectively 98%, 98.6%, 99.1%, the water phase only contains 0.02% n-butyraldehyde, no ionic liquid;
[0031] (2) Put the ionic liquid phase in step (1) in a hydrolysis reactor, add 2ml of water, and perform hydrolysis reaction at 80°C for 50 minutes to form a two-phase product of the ionic liquid phase and the water phase. After testing, the hydrolysis conversion rate of acetal was 100%. The aqueous phase only contains 0.01% n-butyraldehyde and does not contain ionic liquids;
[0032] (3) The ionic liquid phase in step (2) is distilled under reduced pressure, and used for recycling in the reactor. The water phase was distil...
Embodiment 2
[0034] (1) Take 20ml of pretreated 21g / L 1,2,4-butanetriol fermentation broth and put it in the reactor, add [SO4] containing 4mmol n-butyraldehyde 3 HC 8 MIM][HSO 4 ] 10 ml of ionic liquid, the reaction temperatures were 5°C, 25°C, 50°C, the reaction extraction time was 1h, the conversion rates of 1,2,4-butanetriol were 95.1%, 96.3%, 97.0%, and the acetal The recovery rate in the ionic liquid phase is above 99%, and the aqueous phase only contains 0.01% n-butyraldehyde, and does not contain ionic liquid;
[0035] (2) Put the ionic liquid phase in step (1) in a hydrolysis reactor, add 2ml of water, and perform hydrolysis reaction at 80°C for 50 minutes to form a two-phase product of the ionic liquid phase and the water phase. After testing, the hydrolysis conversion rate of acetal was 100%. The aqueous phase only contains 0.01% n-butyraldehyde and does not contain ionic liquids;
[0036] (3) The ionic liquid phase in step (2) is distilled under reduced pressure, and used f...
Embodiment 3
[0038] (1) Take 20ml of pretreated 21g / L 1,2,4-butanetriol fermentation broth and put it in the reactor, add [SO4] containing 4mmol n-butyraldehyde 3 HC 8 MIM][HSO 4 ] ionic liquid 10 ml, the reaction temperature is 25 ℃, the reaction extraction time is 0.5h, 2h, 4h, the conversion rate of 1,2,4-butanetriol is 95.5%, 96.3%, 97.1%, respectively, the acetal in The recovery rate of the ionic liquid phase is above 97%, and the aqueous phase only contains 0.01% n-valeraldehyde and does not contain ionic liquid;
[0039] (2) Put the ionic liquid phase in step (1) in a hydrolysis reactor, add 2ml of water, and perform hydrolysis reaction at 80°C for 50 minutes to form a two-phase product of the ionic liquid phase and the water phase. After testing, the hydrolysis conversion rate of acetal was 100%. The aqueous phase only contains 0.01% n-valeraldehyde and does not contain ionic liquids;
[0040] (3) The ionic liquid phase in step (2) is distilled under reduced pressure, and used ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com