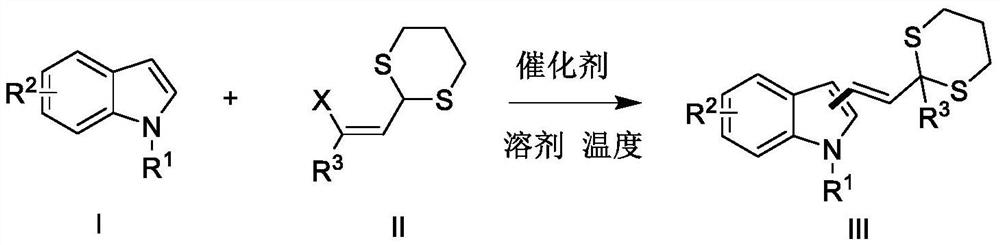
Synthetic method of alkenyl indole derivative
A technology of alkenyl indole derivatives and synthesis methods, which is applied in the field of synthesis of alkenyl indole derivatives, and can solve problems such as environmental hazards and low atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Implementation 1: The reaction steps of the method for preparing (E)-3-(2-(2-methyl-1,3-dithian-2-yl)vinyl)-1H-indole:
[0022] In a 25 mL round bottom flask, add methyl-β-chloro-ethylene-1,3-dithiane (39 mg, 0.2 mmol) and indole (29 mg, 0.25 mmol), and dissolve with 6 mL of 1,2-dichloro After dissolving ethane, add indium tribromide (10.6mg, 0.03mmol) and indium tribromide (6.63mg, 0.03mmol), stir the reaction at room temperature, stop the reaction after TLC detects that the reaction is complete, and the mixture is washed with 1NNaHCO 3 (10 mL) and extracted with EtOAc (15 mL), the organic layer was separated and the aqueous phase was re-extracted with EtOAc (15x3 mL), the combined organic extracts were washed with brine (15x3 mL) and washed in anhydrous Na 2 SO 4 The product was obtained by column chromatography after evaporation of the solvent (yield 82%).
Embodiment 1
[0023] The structure and NMR data of the product (E)-3-(2-(2-methyl-1,3-dithian-2-yl)vinyl)-1H-indole obtained in Example 1 are as follows:
[0024]
[0025] 1 H NMR (400MHz, Chloroform-d) δ8.16(s, 1H), 7.90(d, J=7.0Hz, 1H), 7.37(d, J=7.5Hz, 1H), 7.27(d, J=2.6Hz ,1H),7.27–7.17(m,2H),7.00(d,J=15.9Hz,1H),6.35(d,J=15.9Hz,1H),3.05–2.94(m,2H),2.84–2.72( m,2H),2.07–1.98(m,1H),1.97–1.86(m,1H),1.75(s,3H). 13 C NMR (101MHz, CDCl 3 )δ136.88, 130.73, 125.60, 124.34, 123.85, 122.75, 120.54, 120.26, 114.38, 111.49, 50.98, 30.11, 27.79, 25.06.
Embodiment 2
[0026] Implementation 2: the reaction steps of the method for preparing (E)-2-(2-(2-(tert-butyI)-1,3-dithian-2-yl)vinyl)-3-methyl-1H-indole:
[0027] In a 25ml round bottom flask, add phenyl-β-chloro-ethylene-1,3-dithiane (47mg, 0.2mmol) and 3-methylindole (52.4mg, 0.4mmol) Add indium trichloride (8.8mg, 0.03mmol) after the methyl chloride dissolves, stir the reaction at 50°C, stop the reaction after the reaction is complete as detected by TLC, and wash the mixture with 1N NaHCO 3 (10 mL) and extracted with EtOAc (15 mL), the organic layer was separated and the aqueous phase was re-extracted with EtOAc (15x3 mL), the combined organic extracts were washed with brine (15x3 mL) and washed in anhydrous Na 2 SO 4 The product was obtained by column chromatography after evaporation of the solvent (yield 80%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com