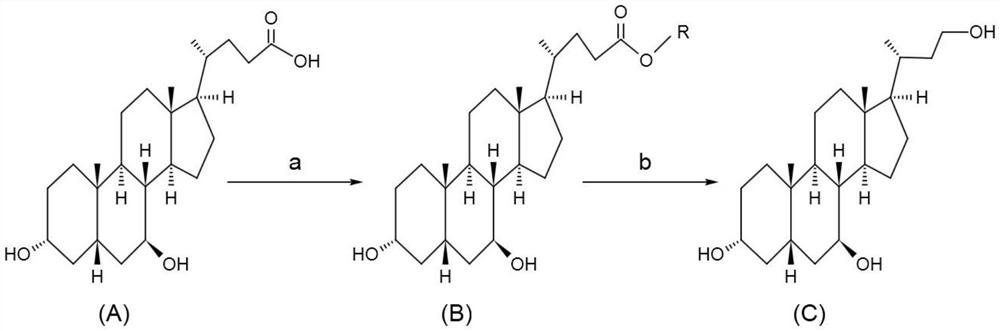
Preparation method of ursodesoxycholic acid EP impurity I
A technology of ursodeoxycholic acid and impurities, applied in the field of medicine, to achieve the effects of high product purity, convenient product quality control, mild and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
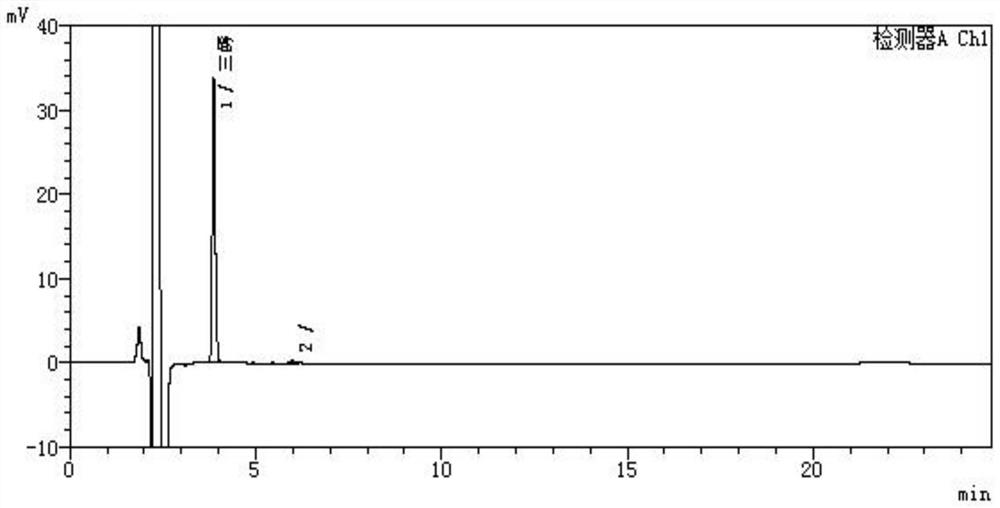
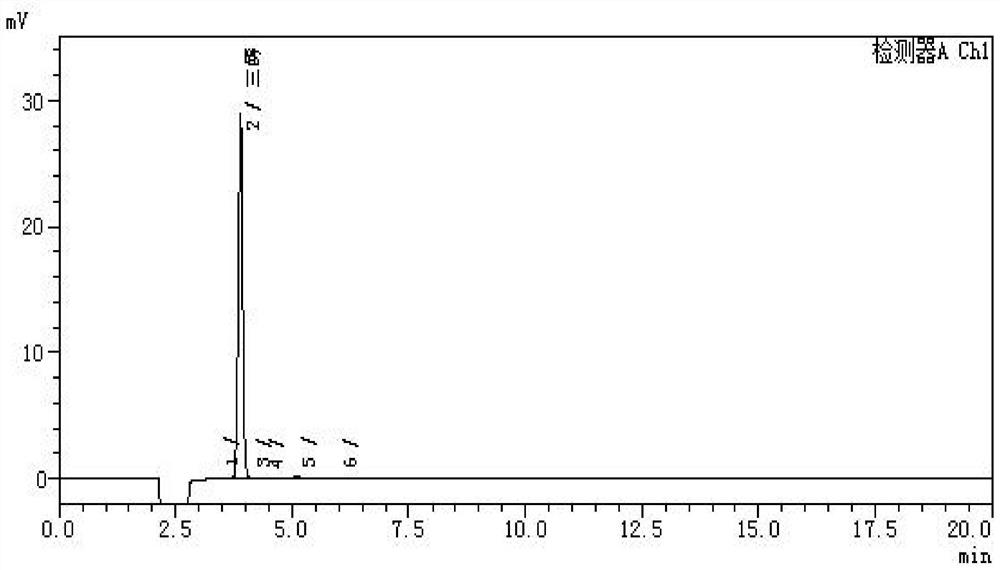
Image
Examples
Embodiment 1
[0025] The preparation method of the ursodeoxycholic acid EP impurity I (UA triol) of the present embodiment comprises the steps:
[0026] (1) Weigh 25g of UDCA, pour it into a 1000ml three-neck flask, add 75ml of methanol, 0.6g of concentrated sulfuric acid, stir and react for 2h, add 60ml of methanol, 90ml of water to cool down to about 0°C, stir for 1h, filter to obtain a wet product, and 100°C Dry for more than 4 hours to obtain 23.4g of 3α,7β-dihydroxy-5β-cholesteric acid methyl ester;
[0027] (2) Add 23.4g of 3α,7β-dihydroxy-5β-methylcholesterate and 530ml of tetrahydrofuran into a 1000ml three-neck flask, stir to dissolve, slowly add 5.96g of lithium aluminum hydride in portions, and keep Stir for about 6 hours;
[0028] (3) Add 23ml of 1:1 hydrochloric acid to quench the reaction, filter, wash the filter cake filtrate with 20ml of tetrahydrofuran at 50-55°C and concentrate in vacuum to a paste, add 180ml of acetone, stir and pulverize, cool to 4°C, stir for 1h, filte...
Embodiment 2
[0030] The preparation method of the ursodeoxycholic acid EP impurity I (UA triol) of the present embodiment comprises the steps:
[0031] (1) Weigh 25g of UDCA, pour it into a 1000ml three-necked flask, add 100ml of ethanol, 2.0g of concentrated hydrochloric acid, stir for 2 hours, add 150ml of ethanol, 90ml of water, cool down to about 0°C, stir for 1h, filter to obtain a wet product, and 100°C Dry for more than 4 hours to obtain 24.25 g of 3α,7β-dihydroxy-5β-cholesteric acid ethyl ester;
[0032] (2) Add 3α,7β-dihydroxy-5β-cholesteric acid ethyl ester and tetrahydrofuran 600ml into a 1000ml three-necked flask, stir to dissolve, cool down to 10°C, slowly add 7.5g lithium aluminum hydride in portions, and stir for about 6 hours.
[0033] (3) Add 30ml of 1:2 hydrochloric acid to quench the reaction, filter, wash the filter cake filtrate with 20ml of tetrahydrofuran at 50-55°C and concentrate in vacuum to a paste, add 220ml of acetone, stir and pulverize, cool to 10-15°C, stir ...
Embodiment 3
[0036] The preparation method of the ursodeoxycholic acid EP impurity I (UA triol) of the present embodiment comprises the steps:
[0037] (1) Weigh 25g of UDCA, pour it into a 1000ml three-neck flask, add 75ml of propanol and 1.0g of p-toluenesulfonic acid, stir for 2 hours, add 150ml of propanol, 90ml of water, cool down to about 0°C, stir for 1 hour, and filter to get wet product, dried at 100°C for over 4 hours to obtain 26.25 g of 3α,7β-dihydroxy-5β-cholesteric acid ethyl ester;
[0038] (2) Add 3α,7β-dihydroxy-5β-cholesteric acid ethyl ester and 700ml tetrahydrofuran into a 1000ml three-necked flask, stir to dissolve, cool down to 10°C, slowly add 7.5g of lithium aluminum hydride in portions, and stir for about 6 hours;
[0039] (3) Add 50ml of 1:10 hydrochloric acid to quench the reaction, filter, wash the filter cake filtrate with 20ml of tetrahydrofuran at 50-55°C and concentrate in vacuum to a paste, add 250ml of acetone, stir and pulverize, cool down to 10-15°C, sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com