Recombinant collagen as well as preparation method and application thereof
A technology for recombining collagen and collagen, applied in the field of collagen expression, can solve the problems of increasing the purification cost, increasing the complexity of the purification process, reducing the yield of full-length α1 chain, etc., and achieving the effect of increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1. Design and synthesis of amino acid sequences
[0073] The amino acid sequence of the human type I collagen α1 chain (denoted as α1(I)) refers to the sequence part 162–1218 (PRO_0000005720) of the Uniprot database P02452-1 (https: / / www.uniprot.org / uniprot / P02452), It is the amino acid sequence of the α1 chain of mature human type Ⅰ collagen, without the signal peptide, C-terminal propeptide, N-terminal propeptide and other parts that will be processed and shed in the α1(Ⅰ) precursor protein. Its sequence is shown in SEQ.ID.NO .1 shown.
[0074] SEQ.ID.NO.1:
[0075] QLSYGYDEKSTGGISVPGPMGPSGPRGLPGPPGAPGPQGFQGPPGEPGEPGASGPMGPRGPPGPPGKNGDDGEAGKPGRPGERGPPGPQGARGLPGTAGLPGMKGHRGFSGLDGAKGDAGPAGPKGEPGSPGENGAPGQMGPRGLPGERGRPGAPGPAGARGNDGATGAAGPPGPTGPAGPPGFPGAVGAKGEAGPQGPRGSEGPQGVRGEPGPPGPAGAAGPAGNPGADGQPGAKGANGAPGIAGAPGFPGARGPSGPQGPGGPPGPKGNSGEPGAPGSKGDTGAKGEPGPVGVQGPPGPAGEEGKRGARGEPGPTGLPGPPGERGGPGSRGFPGADGVAGPKGPAGERGSPGPAGPKGSPGEAGRPGEAGLPGAKGLTGSPGSPGPDGKTGPPGPAGQ...
Embodiment 2
[0108] Embodiment 2. Construction of recombinant expression vector, strain screening
[0109] (1) Construction of recombinant expression vector
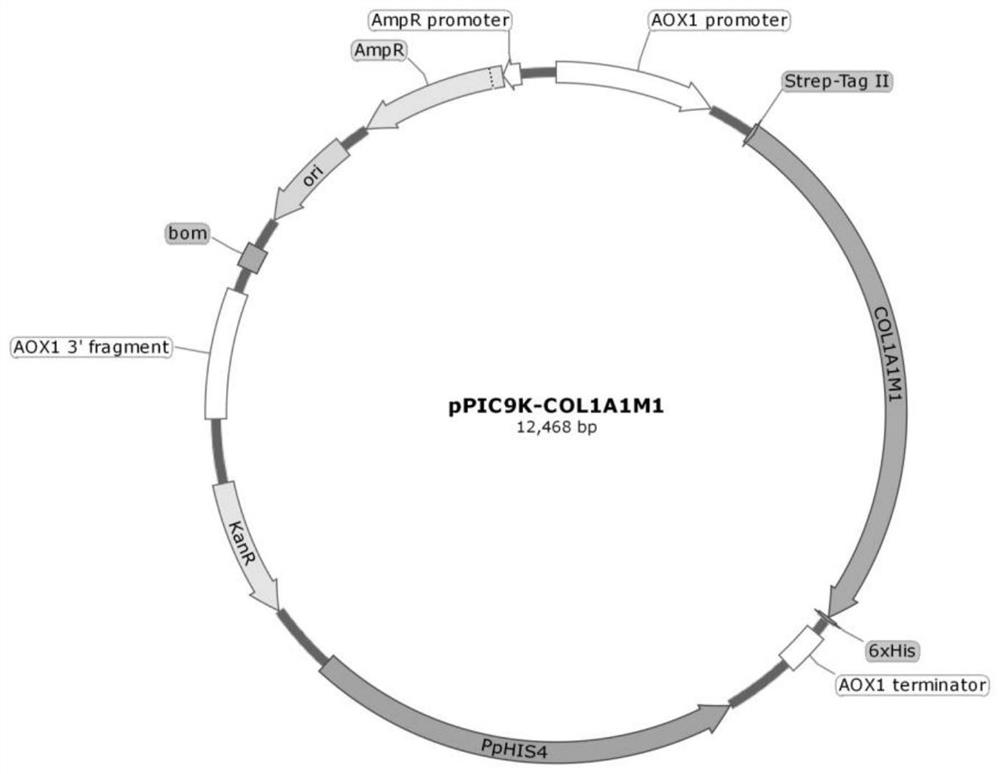
[0110] The synthesized gene fragments SEQ.ID.NO.8 and SEQ.ID.NO.10 were recombined into the pPIC9K empty vector (purchased from Thermo Fisher Scientific Corporation), so that the target fragments could be accurately inserted into the vector containing the secretion signal α- Two recombinant expression vector plasmids, pPIC9K-COL2A1M6 expressing α1(II)M6 and pPIC9K-COL1A1M1 expressing α1(Ⅰ)M1, were obtained in the reading frame of the secretion vector of the factor.
[0111] The pPIC9K-COL2A1M6 and pPIC9K-COL1A1M1 plasmids were transformed into competent Escherichia coli DH5α (purchased from Sangon Bioengineering (Shanghai) Co., Ltd.), positive clones were screened on LB resistance plates containing ampicillin, and recombinant plasmids were extracted for sequencing identification ( Completed by Sangon Bioengineering (Shanghai) Co., L...
Embodiment 3
[0119] Example 3. Induced expression and identification of recombinant collagen
[0120] Take the recombinant engineering bacteria expressing α1(I)M1 and α1(II)M6 obtained in Example 2 respectively, and simultaneously take the engineering strains of Pichia pastoris expressing full-length type I collagen α1 chain protein and expressing full-length II The Pichia pastoris engineering strain of type collagen α1 chain protein was used as a control, and the two control engineering strains were all the previous research results of the inventor's team. The expressed full-length collagen α1 chain also added a Strep-Tag II tag, carboxyl Add 6×His Tag tags at the end), which are respectively from the application number 201911135958.0 (name: yeast recombinant human type I collagen α1 chain protein, synthesis method and application, Pichia pastoris expressing the full-length α1(I) chain in the patent The engineering strains are preserved in the General Microbiology Center of China Microbio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com