Cyclodextrin glycosyltransferase mutant, coding gene and application of cyclodextrin glycosyltransferase mutant
A technology of glycosyltransferase and encoding gene is applied in the application field of biocatalytic preparation of α-glucosyl hesperidin, which can solve the problem that the yield and purity of the product are difficult to balance, and the cyclodextrin glycosyltransferase cannot realize hesperidin. Efficient conversion, affecting the water solubility of products, etc., to achieve the effect of high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
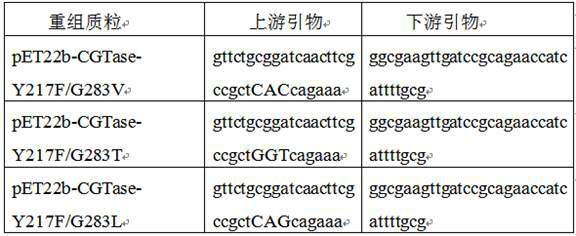
[0017] Example 1: Construction of recombinant engineering bacteria expressing novel cyclodextrin glycosyltransferase mutants
[0018] Refer to the alkalophilic bacillus reported in the NCBI database ( Bacillus sp. ) source of cyclodextrin glucosyltransferase (NCBI accession number NO.AAV38118), add His-tag purification tag, and submit it to Gene Synthesis Company (Hua Da Gene Qinglan Biotechnology Co., Ltd.), after codon optimization , artificially synthesized cyclodextrin glucosyltransferase coding gene (nucleotide sequence shown in SEQ ID NO.1, amino acid sequence shown in SEQ ID NO.2), and cloned into the expression vector pET22b between NcoI / BamHI , to obtain the recombinant plasmid pET22b-CGTase-WT. The primers shown in Table 1 were used to amplify the recombinant plasmid pET22b-CGTase-WT by PCR to obtain recombinant plasmids pET22b-CGTase-Y217F, pET22b-CGTase-G283V, pET22b-CGTase-G283T and pET22b-CGTase-G283L. The PCR reaction system is: 20 μLddH 2 O, 25 μL 2 × Phant...
Embodiment 2
[0026] Example 2: Preparation of novel cyclodextrin glycosyltransferase mutants
[0027] The positive clones described in Example 1 were inoculated on LB liquid medium containing 50 μg / mL ampicillin for resuscitation, 37° C., and after 10 hours of overnight culture in a shaker at 200 rpm, transfected with an inoculum volume concentration of 5%. Inoculated into fresh LB liquid medium containing 50 μg / mL ampicillin, cultured in a shaker at 37°C and 200 rpm for 3 hours; inoculated into the fermentation medium containing 50 mg / L ampicillin with an inoculum of 5% volume concentration , cultured at 35°C for 5h, then lowered the fermentation temperature to 22°C, added glycerol at a rate of 3g / h / L initial fermentation medium, and then added lactose at a rate of 5.4g / h / L initial fermentation medium , where glycerol was added for 15 hours, lactose was added for 5 hours, and the fermentation was continued at 22° C. for 16 hours to obtain a cyclodextrin glycosyltransferase-containing ferm...
Embodiment 3
[0032] Example 3: Application of a fermentation broth containing a novel cyclodextrin glycosyltransferase mutant in the synthesis of α-glucosyl hesperidin
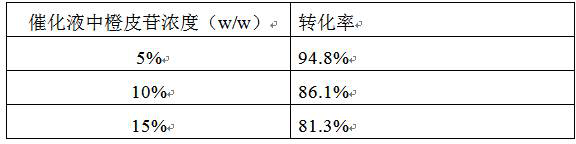
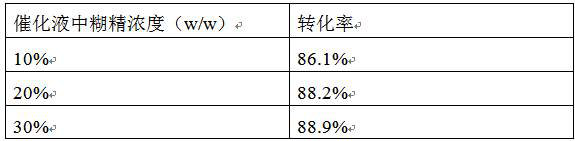
[0033] (1) Catalytic conversion with 10% dextrin dosage and different hesperidin dosage
[0034] According to the method described in Example 2, the fermentation broth containing CGTase-Y217F / G283T cyclodextrin glycosyltransferase mutant, the catalytic process is as follows: take 50mL water and add 10%, 20%, 30% hesperidin respectively, and then add 20% % dextrin to obtain the substrate solution, and the pH was adjusted to 10.0 by adding 2 mol / L NaOH solution dropwise. Dilute the fermentation broth to 2% wet cell concentration and then add 2mol / L NaOH solution dropwise to adjust the pH to 10.0. The substrate solution and fermentation broth were mixed at a volume ratio of 1:1, and catalyzed in a constant temperature water bath magnetic stirrer at 40 °C for 16 h. During the catalytic process, the pH was adjusted by titratin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com