Plasmid for efficiently expressing polypeptide toxin as well as preparation method and application of plasmid
A high-efficiency expression and toxin technology, applied in the field of biomedicine, can solve the problems of less polypeptides and high cost, and achieve the effect of improving soluble expression, high-yield expression and good expression effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
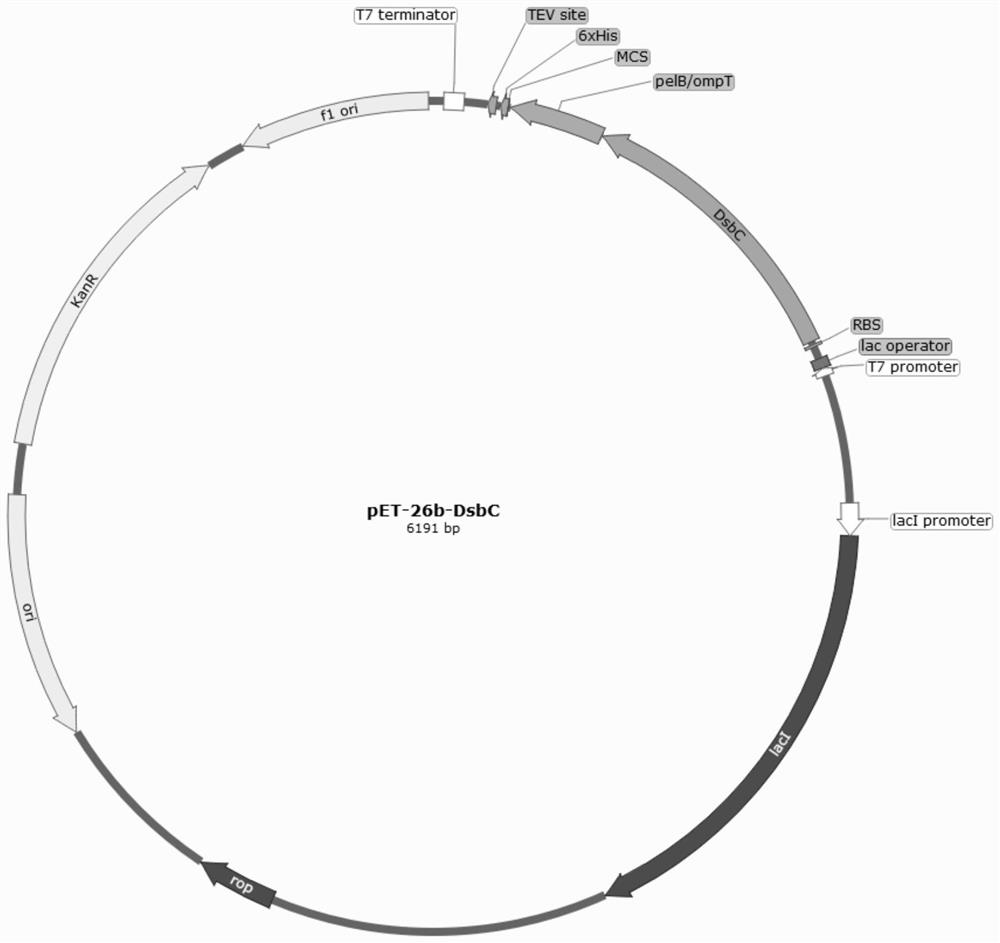
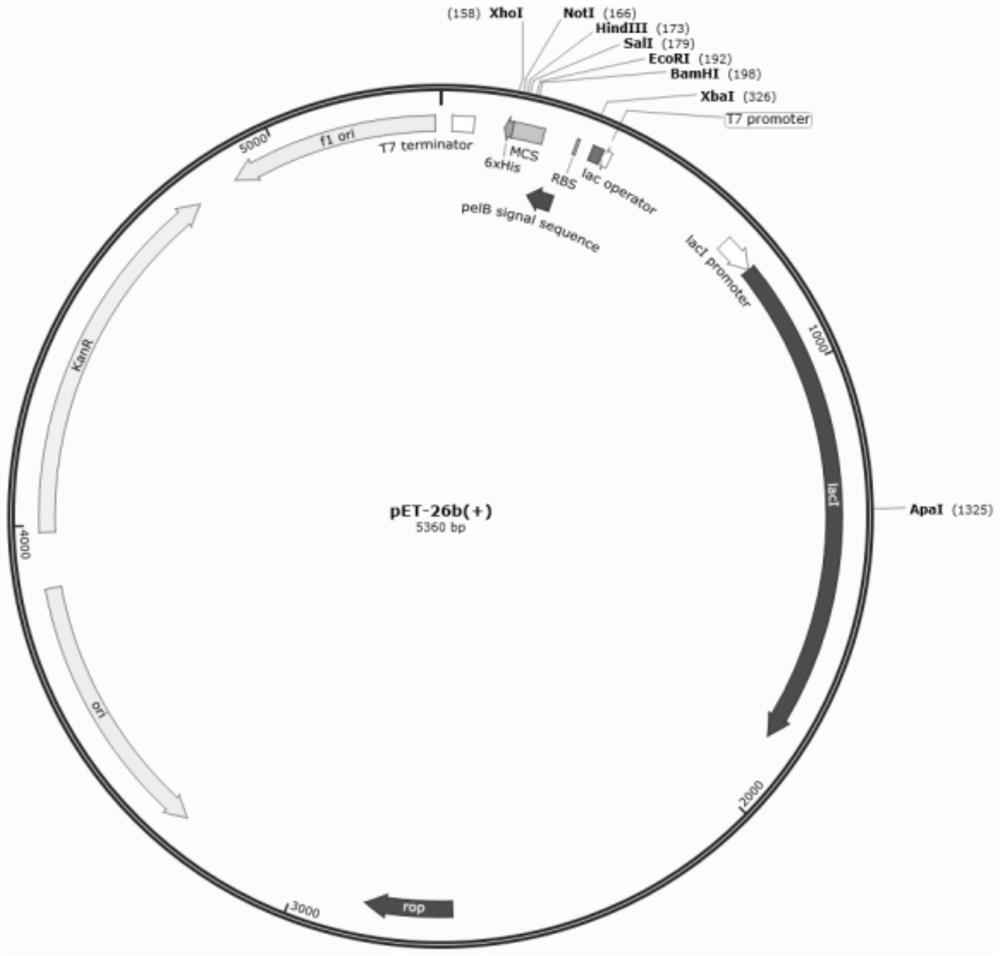
[0034] Construction of pET-26b-DsbC expression plasmid
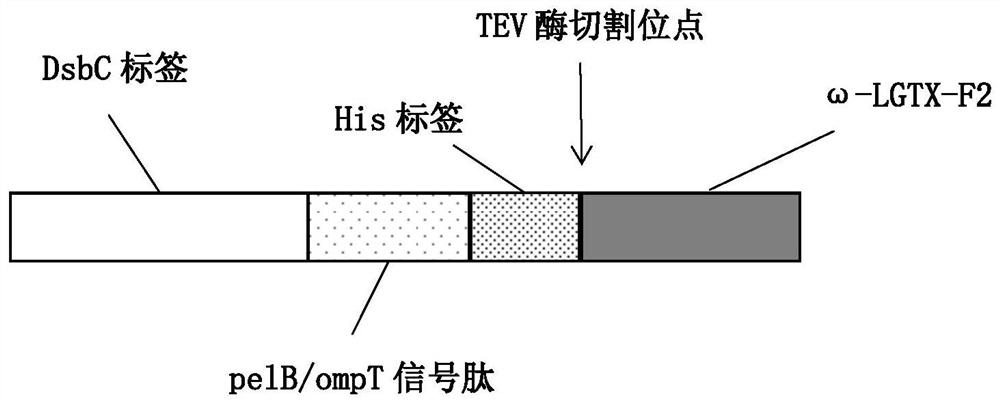
[0035] Insert the DsbC gene with the signal peptide removed into the N-terminal cloning site of the pET-26b vector to obtain the pET-26b-DsbC expression plasmid, the structure of which is shown in figure 1 As shown, it contains T7 promoter, Lac operator, ribosome binding site, DsbC coding region, pelB / ompT, MSC, His coding region, TEV site and T7 terminator.
[0036] The applicant found in the previous research that the polypeptide toxin ω-LGTX-F2 was expressed prokaryotically using the vector pET-32a, and its protein was in the form of an inclusion body, which made it impossible to obtain the recombinant protein by normal means. The polypeptide has 4 pairs of disulfide bonds and needs specific folding processing to form the correct conformation and be successfully expressed. Given the multifaceted properties of its polypeptide toxin, it was investigated whether it could be successfully produced by secretory expression,...
Embodiment 2
[0038] Using the pET-26b-DsbC expression plasmid prepared in Example 1 to prepare spider toxin ω-LGTX-F2 by means of genetic engineering
[0039] (1) Preparation of plasmid expressing ω-LGTX-F2
[0040] According to the codon usage preference of Escherichia coli, the expressed gene series was optimized. In order to enable the TEV enzyme to effectively cut the recombinant protein to remove the fusion tag, a specific recognition coding sequence ENLYFQG was introduced at the N-terminus of ω-LGTX-F2.
[0041] The ω-LGTX-F2 gene (Shanghai Bioengineering Co., Ltd.) was synthesized by chemical method, and then ligated into the same prokaryotic expression vector pET-26b-DsbC (purchased from Novagen) with the same restriction enzyme digestion and added DsbC tag after double digestion with NcoI and XhoI. , DNA sequencing of recombinant plasmids.
[0042] (2) Introduce the plasmid expressing the spider toxin ω-LGTX-F2 into the host expression system
[0043] The successfully constructed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com