Preparation method and application of empagliflozin impurity
A technology for empagliflozin and impurities, applied in the field of preparation of empagliflozin impurities, can solve the problems such as no preparation method reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The present invention provides a kind of preparation method of empagliflozin impurity as shown in formula I, comprises the following steps:
[0030] S1. Dissolve empagliflozin in an organic solvent (including acetonitrile, pyridine) to obtain a mixed solution, cool to -25°C-25°C, wherein the molar ratio of empagliflozin to pyridine is 1:(0.5-2) ;
[0031] S2. Add acetic anhydride dropwise to the mixed solution, wherein the molar ratio of empagliflozin and acetic anhydride is 1:(0.5-2), and the heat preservation reaction is 0.5h-48. After post-treatment, first concentrate under reduced pressure, and then Dissolved with ethyl acetate, washed with water at least twice, and then purified by silica gel column chromatography after vacuum distillation of the obtained organic phase, wherein the silica gel was selected from 200 to 300 mesh, and the eluent was V (dichloromethane / methanol) = 20 / 1 mixed solvent; thereby obtaining Empagliflozin impurities.
[0032] The reaction f...
Embodiment 1
[0037] The present embodiment provides a kind of preparation method of empagliflozin impurity, concrete steps are as follows:
[0038] S1. Dissolve 2.7g of Empagliflozin (6.0mmol) in 50mL of acetonitrile and 0.50g of pyridine (6.3mmol) to obtain a mixed solution, and cool to 0°C in an ice-water bath;
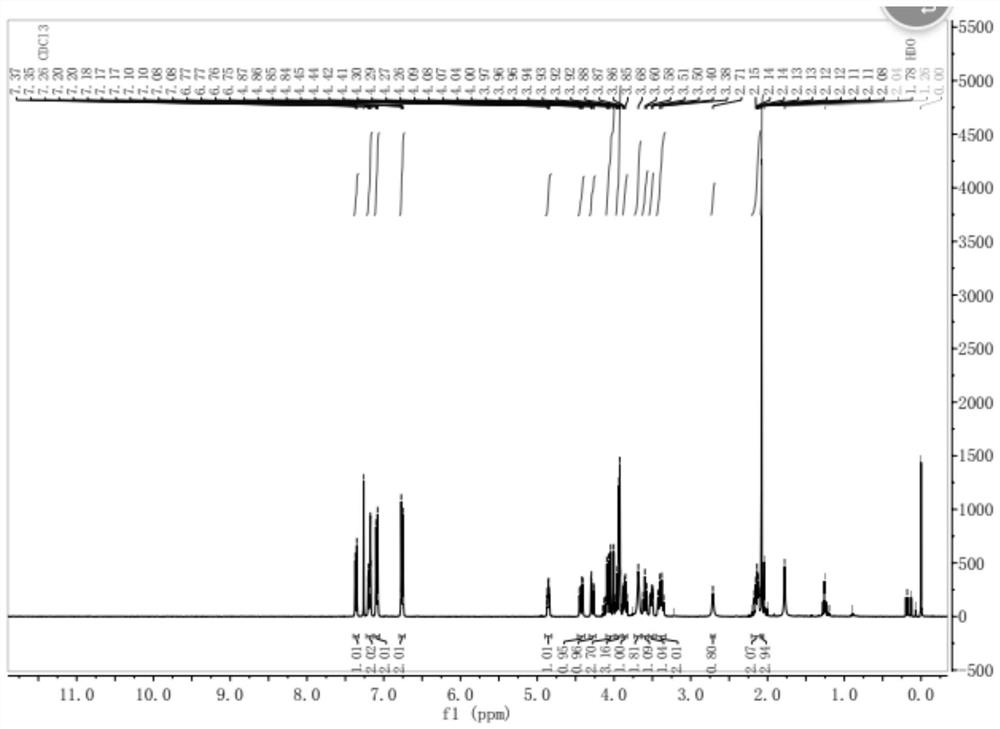
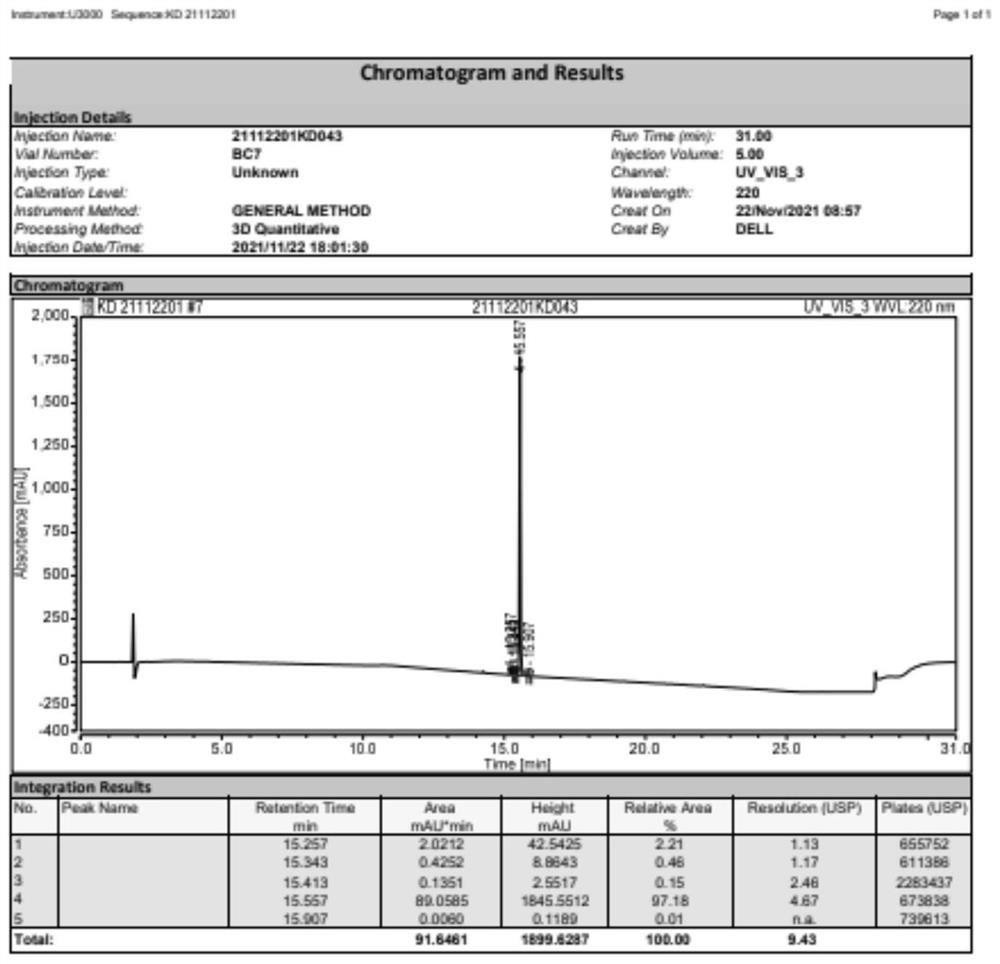
[0039] S2. Add 0.65 g of acetic anhydride (6.4 mmol) dropwise to the above mixed solution, keep the temperature for 3 hours, and after distillation under reduced pressure, dissolve the residue with 50 mL of ethyl acetate, wash twice with water, and obtain the organic phase after distillation under reduced pressure , and then purified by silica gel column chromatography, the silica gel is selected from 200 to 300 meshes, the eluent: DCM / MeOH=20 / 1, and then through rotary evaporation, to obtain the empagliflozin impurity shown in formula I, white solid 1.82g , yield 61.6%, HPLC purity 97.18%. 1 H NMR(400MHz,Chloroform-d)δ7.36(d,J=8.0Hz,1H), 7.23-7.14(m,2H),7.13-7.05(m,2H),6.80-6....
Embodiment 2
[0043] This embodiment provides a preparation method of empagliflozin impurities, the specific steps are basically the same as in Example 1, the difference is that the pyridine added in the S1 step is 0.237g (3mmol), and the finally obtained white solid is 0.65 g, the yield is 22%; wherein, the molar ratio of empagliflozin to pyridine is 1:0.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com