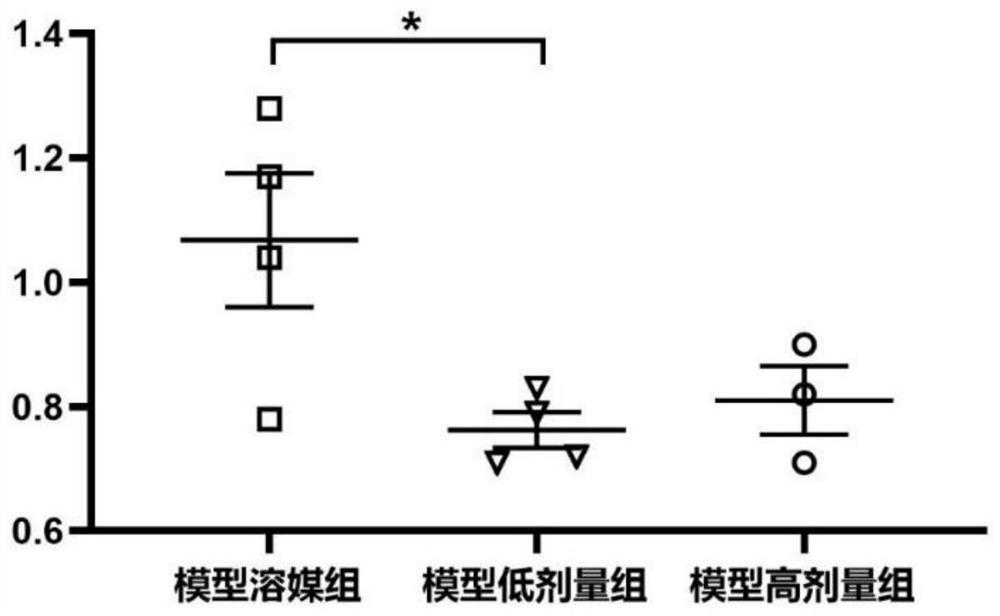
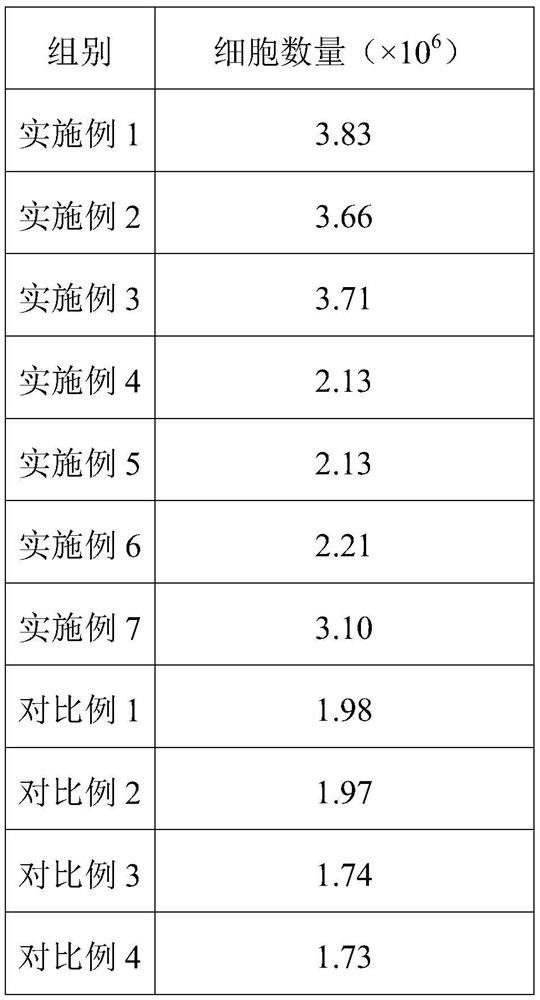
Human umbilical cord mesenchymal stem cell culture medium, human umbilical cord mesenchymal stem cell injection, preparation method and application of human umbilical cord mesenchymal stem cell injection in preparation of medicine for treating cerebral apoplexy
A technology of mesenchymal stem cells and human umbilical cord, applied in the direction of cell culture active agents, biochemical equipment and methods, animal cells, etc., can solve the problems of limited treatment strategies and affect the treatment effect, to facilitate mass spectrometry detection, promote proliferation ability, and promote proliferating effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
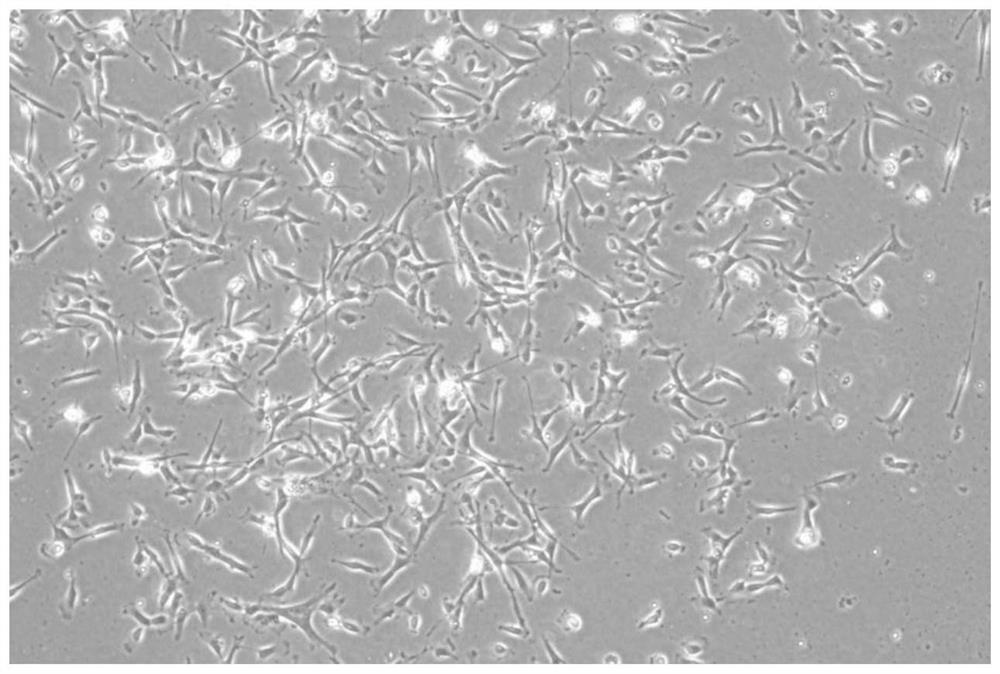
[0065] This embodiment provides a method for culturing human umbilical cord mesenchymal stem cells
[0066] (1) Prepare serum-free medium according to the following formula. There is no strict order for the addition of various components, and it can be operated at room temperature. The preparation process does not need to be heated, and it is carried out under a biological safety cabinet. After the preparation is completed, use a 0.22 μm filter. filter.
[0067] The formula is: DMEM / F12 medium, platelet-derived growth factor (PDGF) 20ng / ml, epidermal growth factor (EGF) 15ng / ml, vitamin C 25μg / ml, insulin 12μg / ml, β-mercaptoethanol 10ng / ml, SAG 7μmol / L, folic acid 5.5mg / L, hypoxia-inducible factor-1 (HIF-1) 15ng / ml, cobalt protoporphyrin (CoPP) 50μmol / L, basic fibroblast growth factor (bFGF) 10mg / ml , Simvastatin 10 μg / ml.
[0068] (2) Shred the isolated Wharton's jelly with surgical scissors, put it in a collagenase solution with a concentration of 0.05U / ml for digestion fo...
Embodiment 2
[0070] This embodiment provides a method for culturing human umbilical cord mesenchymal stem cells
[0071] (1) Prepare serum-free medium according to the following formula. There is no strict order for the addition of various components, and it can be operated at room temperature. The preparation process does not need to be heated, and it is carried out under a biological safety cabinet. After the preparation is completed, use a 0.22 μm filter. filter.
[0072]The formula is: F12 medium, platelet-derived growth factor (PDGF) 12ng / ml, epidermal growth factor (EGF) 25ng / ml, vitamin C 20μg / ml, insulin 15μg / ml, β-mercaptoethanol 6ng / ml, SAG 10μmol / L, folic acid 3.5mg / L, hypoxia-inducible factor-1 (HIF-1) 18ng / ml, cobalt protoporphyrin (CoPP) 30μmol / L, basic fibroblast growth factor (bFGF) 15mg / ml, octyl Vastatin 6 μg / ml.
[0073] (2) Cut the isolated Wharton's jelly into pieces with surgical scissors, put it in a collagenase solution with a concentration of 0.06U / ml for digest...
Embodiment 3
[0075] This embodiment provides a method for culturing human umbilical cord mesenchymal stem cells
[0076] (1) Prepare serum-free medium according to the following formula. There is no strict order for the addition of various components, and it can be operated at room temperature. The preparation process does not need to be heated, and it is carried out under a biological safety cabinet. After the preparation is completed, use a 0.22 μm filter. filter.
[0077] The formula is: DMEM / F12 medium, platelet-derived growth factor (PDGF) 25ng / ml, epidermal growth factor (EGF) 10ng / ml, vitamin C 30μg / ml, insulin 10μg / ml, β-mercaptoethanol 12ng / ml, SAG 6μmol / L, folic acid 7mg / L, hypoxia-inducible factor-1 (HIF-1) 12ng / ml, cobalt protoporphyrin (CoPP) 70μmol / L, basic fibroblast growth factor (bFGF) 7mg / ml, Simvastatin 13 μg / ml.
[0078] (2) Cut the isolated Wharton's jelly with surgical scissors, place it in a collagenase solution with a concentration of 0.05U / ml for digestion for 3h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com