Lateolabrax japonicus FGF6A, FGF6B and FGF18 recombinant protein as well as preparation method and application thereof
A technology of FGF6B and recombinant protein, applied in botany equipment and methods, biochemical equipment and methods, applications, etc., can solve research blanks and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Construction of perch FGF6A, FGF6B and FGF18 expression vector pET-28a(+)-SUMO-FGF
[0037] Using perch muscle cDNA as a template, PCR was performed using primers FGF6A-F / R, FGF6B-F / R, FGF18-F / R (Shenggong, Shanghai) and reagent 2×Phanta Max Master Mix (Novizant, Nanjing) The DNA sequences of the open reading frames (ORF) of the perch FGF6A, FGF6B and FGF18 genes were amplified. It was ligated into pCE2 TA / Blunt Zero vector (Novazyme, Nanjing) by TOPO ligation to transfect DH5α Escherichia coli, a single colony was screened on ampicillin solid medium, and Sanger sequencing and NCBI BLAST were used to identify whether the sequence was correct. Using the above-mentioned monoclonal cultures of perch FGF6A, FGF6B and FGF18 as templates, PCR amplification was performed using primers PE-FGF-F / R and 2×Phanta Max Master Mix to obtain mature FGF6A, FGF6B and FGF18 with homology arms. The double-stranded DNA fragment corresponding to the peptide; meanwhile, the pET-28a(...
Embodiment 2
[0041] Example 2: Preparation of sea bass FGF6A, FGF6B and FGF18 Escherichia coli expression strains
[0042] The expression vector pET-28a(+)-SUMO-FGF was transfected into BL21(DE3) competent Escherichia coli (Ang Yu, Shanghai), and cultured with kanamycin-resistant LB solid medium at 37°C overnight to screen a single colony, namely The engineering strain pET-28a(+)-SUMO-FGF-BL21(DE3) was used to express the prokaryotic expression of FGF6A, FGF6B and FGF18 of perch. Pick a single colony and culture it overnight in 10 mL of kanamycin-resistant LB liquid medium at 37°C and 220 rpm, and then inoculate it in 100 mL of kanamycin-resistant LB liquid medium at a ratio of 1:100. Cultivate at 220 rpm until OD600 reaches 0.4-0.5, then add IPTG to make the final concentration 0.15mmol / L (take out 50mL of bacterial liquid as a control before induction), at 20℃, 120 rpm and culture for 12h, 4000× Centrifuge at g for 10 min to collect the bacteria, discard the supernatant, resuspend the b...
Embodiment 3
[0043] Example 3: Soluble expression and protein purification of sea bass FGF6A, FGF6B and FGF18 recombinant proteins
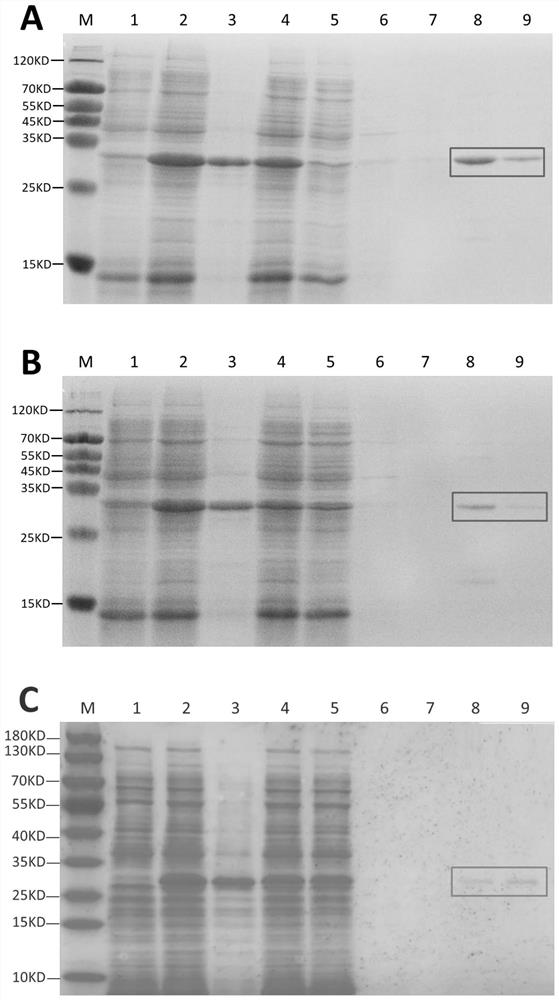
[0044] Inoculate in 500mL kanamycin-resistant LB liquid medium at a ratio of 1:100, culture at 37°C, 220 rpm until OD600 reaches 0.4-0.5, then add IPTG to make the final concentration 0.15mmol / L, 20 Centrifuge at 120 rpm for 12 hours, then centrifuge at 4000×g for 10 minutes to collect the bacteria, discard the supernatant, resuspend the bacteria in 50 mL of non-denaturing lysis buffer, add lysozyme at a final concentration of 1 mg / mL, and lyse on ice for 30 minutes, 60W Sonicate for 15 min, ultra-sonicate for 5 s and stop for 5 s, centrifuge at 12,000×g for 10 min to discard the precipitate, retain the supernatant, filter with a 0.45 μm or 0.22 μm filter membrane to remove insoluble particles, and use TED-Ni filler (Abbison, Shanghai) for His Tag affinity purification to obtain FGF6A, FGF6B and FGF18 recombinant proteins ( image 3 Middle sample 8-9), can b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com