Preparation method of brimonidine tartrate impurity E
A technology for brimonidine tartrate and impurities, which is applied in the field of preparation of pharmaceutical impurity standard substances, can solve problems such as shortage of impurity reference substances, and achieves the effects of short preparation period, simple operation and reasonable design.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of Intermediate Compound V
[0030] Type III (4.0 g, 17.8 mmol), IV (5.2 g, 17.8 mmol), silver (4.5 g, 17.8 mmol) and dimethyl sulfoxide (60 mL). The nitrogen was protected from temperature to 50 ° C, and the mixture was stirred for 20 hours. After lowering to room temperature, the reaction solution was poured into ice water, ethyl acetate (20 mL) was added twice, combined with the organic layer, saturated brine, washed twice, dry sodium sulfate, remove the solvent, residue silicone column layer Analysis was purified to give intermediate compound V (4.2 g, 11.5 mmol), yellow solid, yield of 64.6%.
Embodiment 2
[0031] Example 2: Preparation of lychane monogamonic impurity E
[0032] The intermediate compound V (1.83 g, 5.0 mmol) was dissolved in dichloromethane (27 mL), and trifluoroacetic acid (5.7 g, 50 mmol) was added under an ice bath, and then the mixture was stirred at room temperature 6 h. The solvent was concentrated, and the residue was purified by the reversed rapid column to obtain a tartarid monogamodic impurity E (1.20 g, 4.5 mmol), pale yellow solid, yield 90%.
[0033] The molecular formula of tartrate monogamodic impurity E: c 9 Hide 8 BRN 5 ;
[0034] The molecular weight of the tartrate monogamodic impurity E is: 266.10.
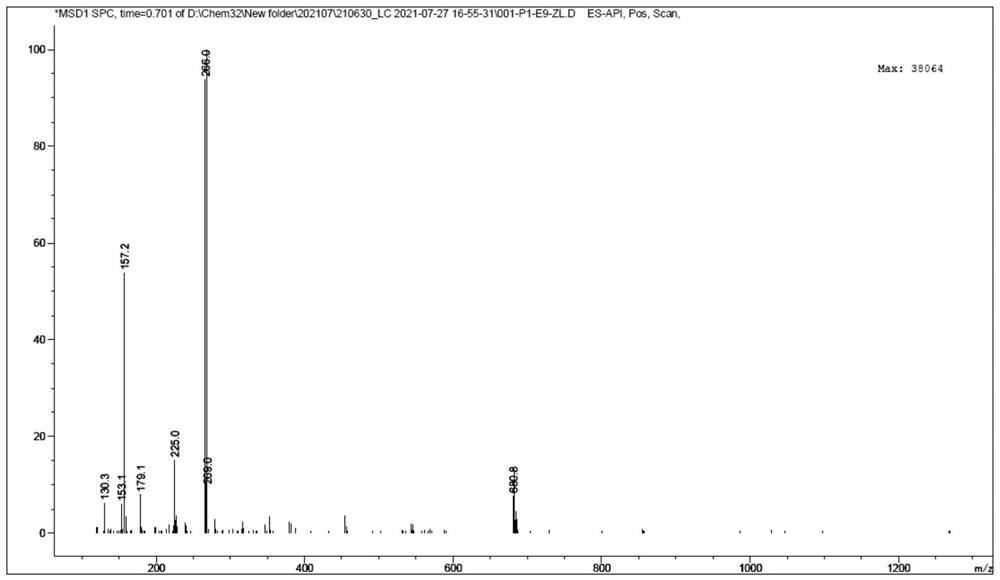
[0035] The mass spectrometer is used to analyze the synthetic product tartarid monogamonic impurity E E, and its MS spectrum is figure 1 As shown in the mass spectrum, the peak of m / z = 266.0 is the molecular ion peak M + H of 酒 莫 莫 定 E + , Consistent with the molecular weight of the lytectate monogamine E.
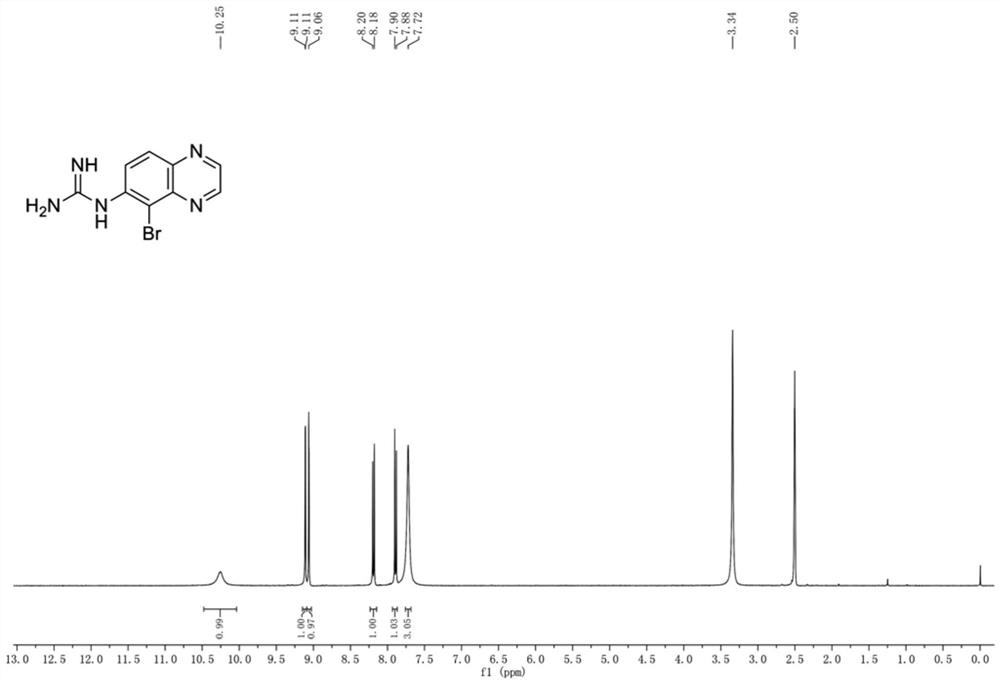
[0036] The nuclear magnetic resonance un...
Embodiment 3
[0042] Example 3: Preparation of Intermediate Compound V
[0043] To the 250 ml of three bottles, III (4.0 g, 17.8 mmol), IV (5.2 G, 17.8 mmol), silver fluorinated (2.26 g, 17.8 mmol) and N-methylpyrrolidone (40 mL) were added. The nitrogen removal was raised to 70 ° C, and the reaction was stirred for 16 hours. After lowering to room temperature, the reaction solution was poured into ice water, ethyl acetate (20 mL) was added twice, combined with the organic layer, saturated brine, washed twice, dry sodium sulfate, remove the solvent, residue silicone column layer Purification was purified to obtain an intermediate compound V (3.8 g, 10.4 mmol), yellow solid, yield of 58.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com