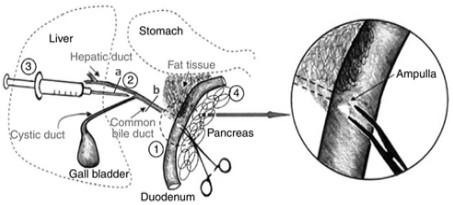
Method for promoting transdifferentiation of pAdM3C infected rat pancreatic duct cells
A pancreatic duct and transdifferentiation technology, applied in the direction of pancreatic cells, cell dissociation methods, biochemical equipment and methods, etc., can solve the problems of difficult culture, complicated identification, low cell acquisition rate, etc., and achieve the goal of increasing the number and purity of cells Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
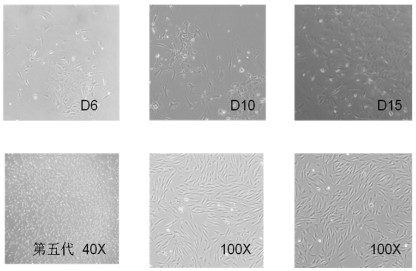
[0051] Example 1 Separation and purification of rat pancreatic ductal cells
[0052] 1. Preparation 2-3 days before the experiment
[0053] 1 Islet Isolation Reagent:
[0054] 1) Hanks solution: (Gibco, 14025) 500 ml / bottle, or prepare Ca-free 2+ , Mg 2+ Hanks solution, stored at 4°C after sterilization.
[0055] 2) 25% BSA (Bovine Serum Albumin): 1ml aliquot and store at -20°C.
[0056] 3) 12.5mg / ml collagenase storage solution: (collagenase type IX, Sigma, C-7657-1G) 1ml aliquots, store at -20°C.
[0057] 4) 0.67g / l DTZ stain stock solution: aliquot 50μl and store at -20°C.
[0058]5) Citrate buffer: citric acid (Citric acid, Sigma, C2404-100G), filter sterilized, aliquot 1ml, store at -20°C.
[0059]
[0060] Pancreatic ductal cell isolation reagents:
[0061] 1) 1×PBS-CMF: calcium and magnesium free, cell grade (that is, PBS in this protocol) (about 200ml / rat)
[0062] 2) 0.025% trypsin: 0.25% trypsin-EDTA, diluted 10 times with PBS.
[0063]
[0064] 3) Med...
Embodiment 2
[0123] Example 2 Transdifferentiation induction using adenovirus (pAd-M3C)
[0124] Experimental steps:
[0125] 1 plate: Inoculate a 12-well plate, 2.5×10 per well 5 The pancreatic ductal cells prepared in Example 1.
[0126] 2 After 24 h, the culture medium was removed, and 500 μl of fresh medium c was added to each well.
[0127] 3 Convert MOI to 20, 30, 50, 100, and 200 μl corresponding volumes of adenovirus (pAd-M3C purchased from Addgene) virus supernatant volume, respectively added in the following table.
[0128]
[0129] 4 Shake the culture plate continuously and slowly, and incubate for 3 h.
[0130] 5 Add 1.5 ml of fresh medium c and culture for 72 h.
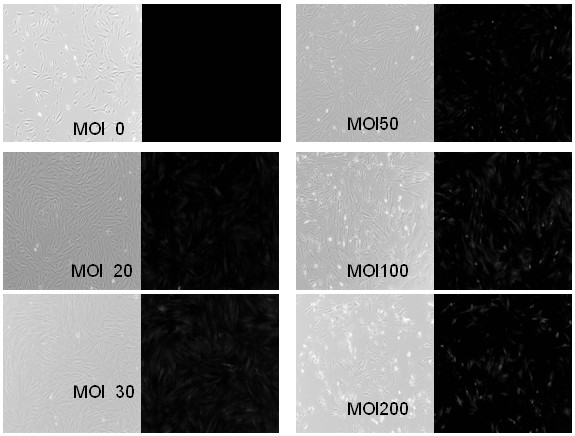
[0131] image 3 It is to observe the situation of pancreatic ductal cells after infection under a microscope, in which the red fluorescence represents the cells that are infected and express the reporter gene. It can be seen from the figure that the pancreatic ductal cells were infected with adenovirus (pAd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com