Kit for detecting thyroglobulin antibody and subtype thereof
A technology of thyroglobulin and kits, applied in the field of biomedicine, can solve the problems of not being included in clinical routine testing, narrow linear range, and toxic media
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. Detection of thyroglobulin antibody and its subtypes
[0029] 1. Determination of serum TGAb total IgG:
[0030] (1) Antigen dilution: Take a 15mL centrifuge tube and dilute with 6mL of pH 7.4 antigen buffer 125 I-TG to 20000CPM / 60μL;
[0031] (2) Incubate the labeled antigen with the serum overnight: Take a 96-well plate, add 5 μL of the serum to be tested in each well, and then add 60 μL of the diluted labeled antigen to each well. The CPM value of each well should be ≥20000. Each sample and The quality control serum is double-well, the labeled antigen and the serum are shaken and mixed at room temperature for 1 hour, and then overnight at 4°C;
[0032] (3) Incubate 96-well PVDF plate: Take a 96-well PVDF micropore filter plate, add 150 μL of antigen buffer to each well, and overnight at 4°C;
[0033] (4) Precipitate antigen-antibody complexes: Discard the incubation solution of the 96-well PVDF plate, add 25 μL of protein A / G-agarose to each well, and transfer ...
Embodiment 2
[0062] Comparison of agreement and correlation with electrochemiluminescence kits
[0063] 99 human serum samples were detected simultaneously with MODMLAR ANALYTICS E170 automatic electrochemiluminescence immunoassay system and supporting kits (Roche Diagnostics, Germany). The TGAb test results of 99 outpatients were classified into negative and positive results, as shown in Table 4.
[0064] Table 4 The results of two methods for measuring TGAb
[0065]
[0066] The kit of the present invention detects a positive coincidence rate of 100%, a negative coincidence rate of 79.2%, a total coincidence rate of 94.9%, and a Kappa value of 0.852, suggesting that the test results of the kit of the present invention have a high degree of consistency with the ECLIA method, and there is no significant difference Significance (paired chi-square test, P>0.05). Carry out Spearman correlation analysis to the TGAb measurement value in 80 parts of detection limit scope (10-4000IU / mL), it ...
Embodiment 3
[0068] Determination of each IgG subtype of TGAb
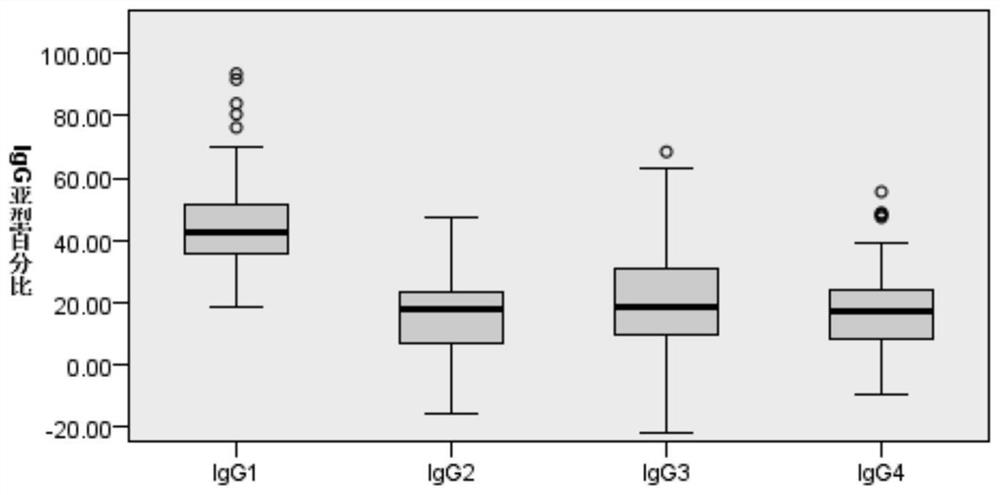
[0069] Detection of IgG subtypes of TGAb in serum of patients with Hashimoto's thyroiditis. According to the thyroid function status at the first diagnosis, they were divided into 3 groups: hypothyroid group, subclinical hypothyroid group (subclinical hypothyroid group), normal thyroid function group (normal thyroid function group).
[0070] figure 2 It is the distribution map of TGAb IgG subtype in the serum of patients with Hashimoto's thyroiditis (84 cases). figure 2 The results showed that in patients with Hashimoto's thyroiditis, there were statistical differences in the distribution of IgG subtypes of TGAb, and IgG1 was the main one (IgG1%: IgG2%: IgG3%: IgG4% = 42.42: 17.76: 18.44: 16.92).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com