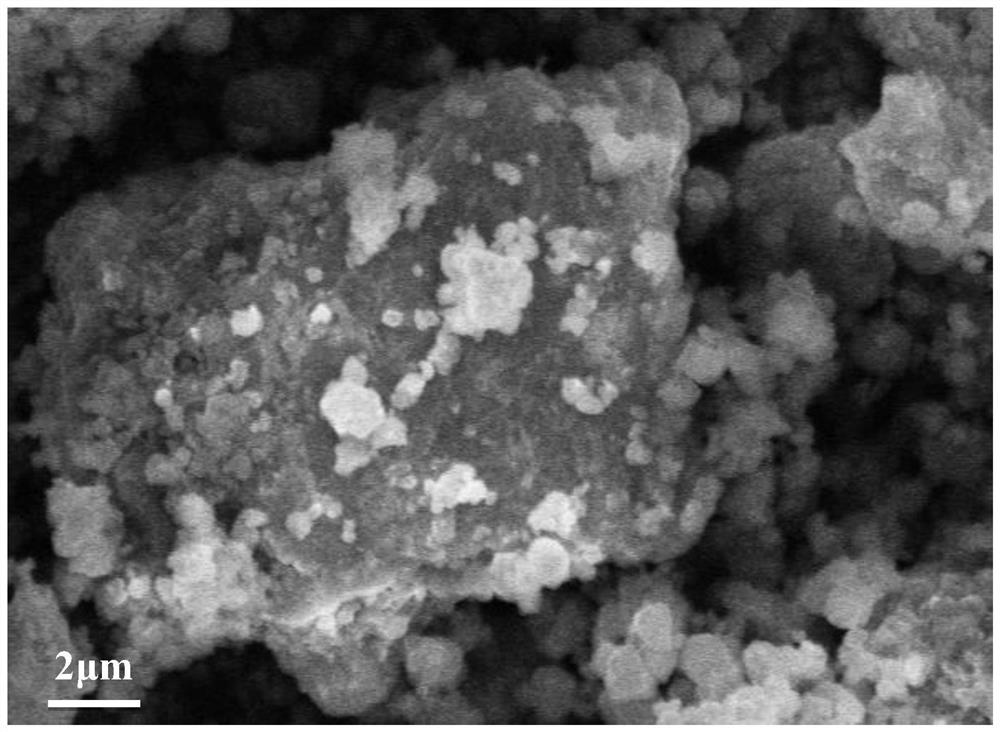
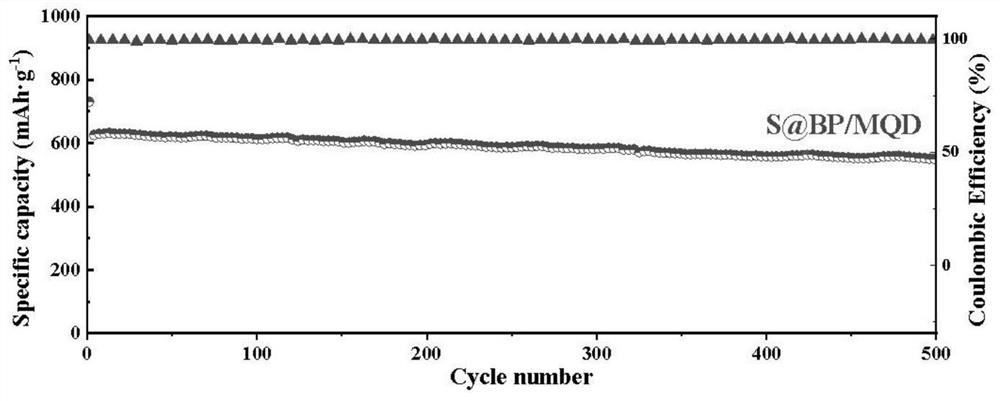
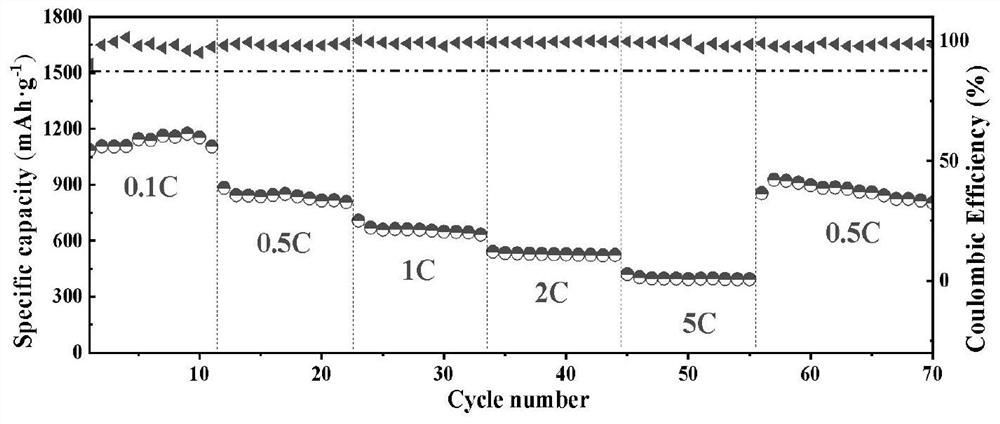
Preparation method and application of lithium-sulfur battery positive electrode material
A technology for lithium-sulfur batteries and cathode materials, which is applied in the field of material science and can solve the problems of low electrical conductivity, low cycle performance of lithium-sulfur batteries, and complicated charging and discharging processes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Mix black phosphorus powder and magnesium boride powder in a mass ratio of 2:3, put them in a beaker and mix for 24 hours.
[0038] (2) Put it into a crucible after drying, and use a muffle furnace to perform high-temperature calcination at 620°C with a heating rate of 10°C / min and a holding time of 24h.
[0039] (3) Take out the calcined product, wash the product centrifugally with deionized water, and then dry it at 60° C. to obtain black phosphorus@magnesia quantum dots.
[0040] (4) Mix and grind the black phosphorus@magnesia quantum dots obtained by drying with elemental sulfur powder in a mass ratio of 1:3 for 15 minutes, and mix the obtained product with carbon disulfide in a mass ratio of 1:5, and continue grinding until The carbon disulfide is completely evaporated to obtain a uniformly mixed product, which is then collected in a sealed weighing bottle, and finally placed in an oven at 155°C for 10 hours. Finally, samples of black phosphorus@magnesia quant...
Embodiment 2
[0045] (1) Mix black phosphorus powder and magnesium boride powder at a mass ratio of 1:1, put them into a beaker, add deionized water and stir for 24 hours.
[0046] (2) Put it into a crucible after drying, and use a muffle furnace to perform high-temperature calcination at 570° C., the heating rate is 10° C. / min, and the holding time is set to 24 hours.
[0047] (4) Take out the calcined product, wash the product centrifugally with deionized water, and then dry it at 60° C. to obtain the black phosphorus / magnesia quantum dots.
[0048](5) Mix and grind the black phosphorus@magnesia quantum dots obtained by drying with the elemental sulfur powder in a mass ratio of 1:3 for 15 minutes, and mix the obtained product with carbon disulfide in a mass ratio of 1:5, and continue grinding until The carbon disulfide is completely evaporated to obtain a uniformly mixed product, which is then collected in a sealed weighing bottle, and finally placed in an oven at 150°C for 12 hours. Fin...
Embodiment 3
[0050] (1) Mix black phosphorus powder and magnesium boride powder at a mass ratio of 1:7, put them into a beaker, add deionized water and stir for 24 hours.
[0051] (2) Put it into a crucible after drying, and use a muffle furnace to perform high-temperature calcination at 720°C with a heating rate of 10°C / min and a holding time of 1h.
[0052] (4) Take out the calcined product, wash the product centrifugally with deionized water, and then dry it at 60° C. to obtain black phosphorus@magnesia quantum dots.
[0053] (5) The black phosphorus / magnesia quantum dots obtained by drying are mixed and ground for 15 minutes with the elemental sulfur powder in a ratio of 1:3 by mass, and the product obtained is mixed with carbon disulfide in a ratio of 1:5 by mass, and then continue to grind until The carbon disulfide evaporates completely to obtain a uniformly mixed product, which is then collected in a sealed weighing bottle, and finally placed in an oven at 160°C for 4 hours. Final...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com