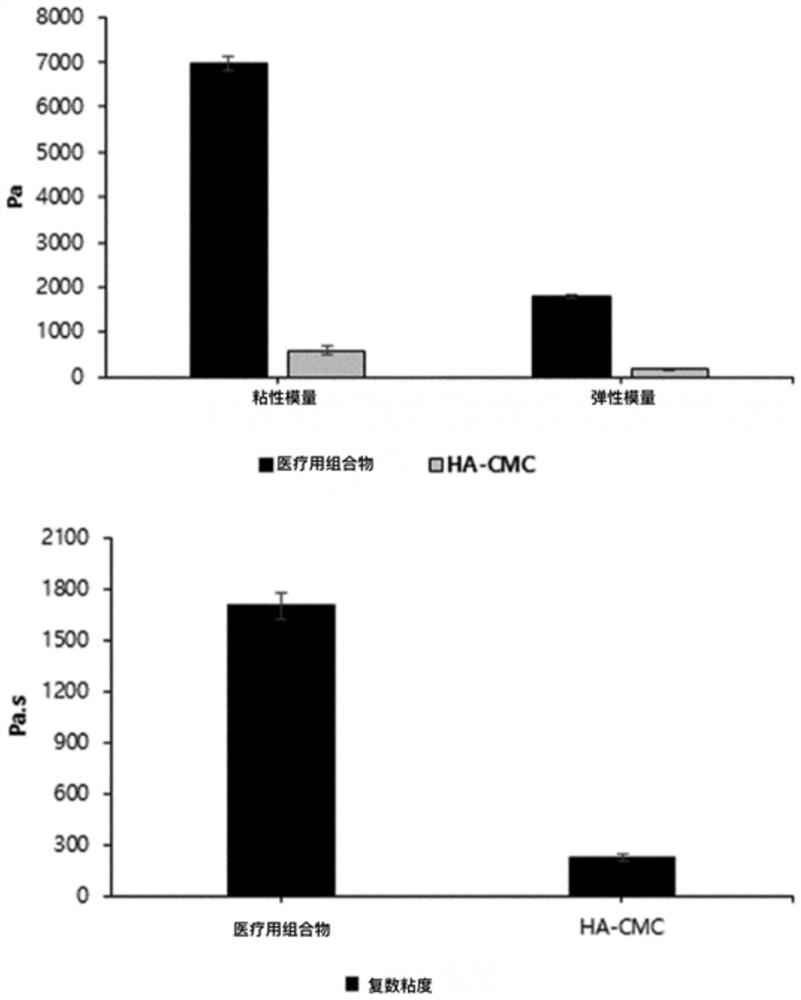
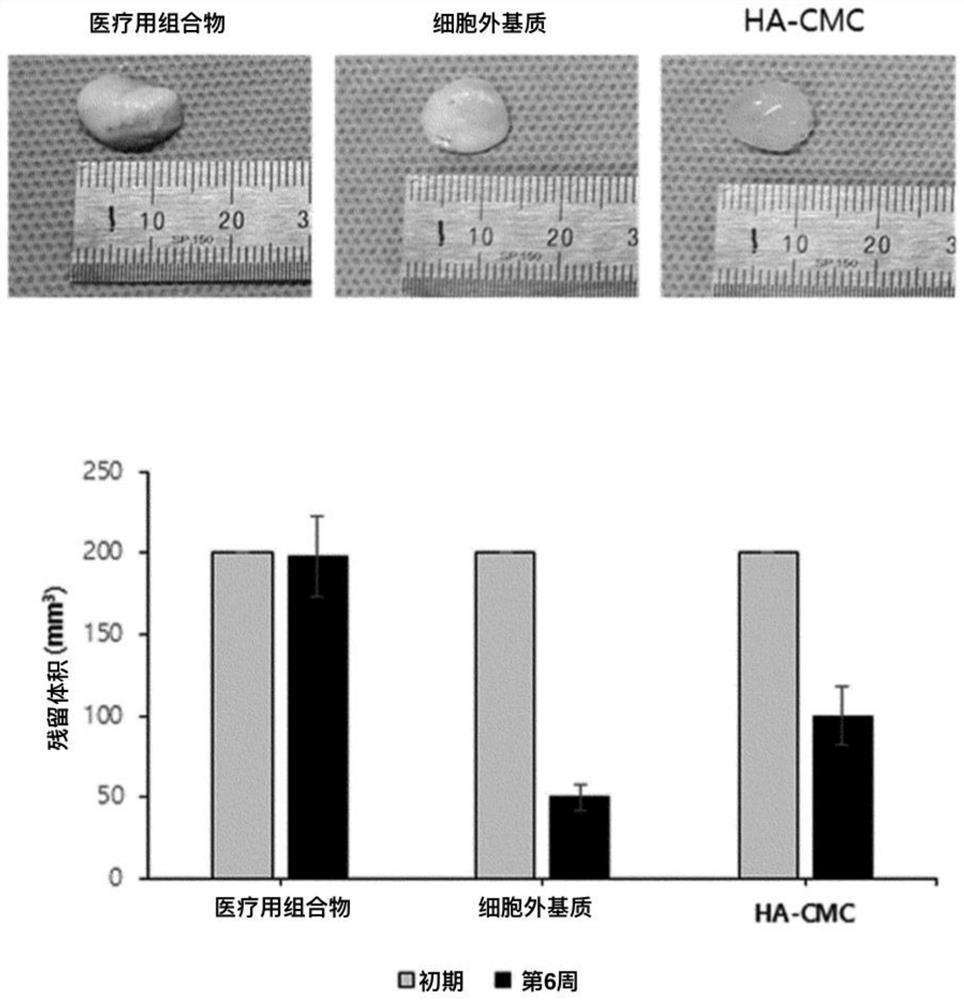
Medical composition comprising adipose tissue-derived extracellular matrix and method for producing same
A kind of technology of adipose tissue and manufacturing method, which is applied in the fields of pharmaceutical formulation, medical science, drug delivery, etc., can solve the problems of volume maintenance, etc., and achieve the effect of improving viscoelastic properties and excellent volume maintenance in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0094] Hereinafter, the present invention will be described more specifically by way of examples. However, the scope of the present invention is not limited to the following examples, and those skilled in the art should understand that various variations, modifications, or applications can be made within the range derived from the ideas described in the claims without departing from the technical ideas.
Embodiment 1
[0096] Example 1. Medical composition in which human adipose tissue-derived extracellular matrix particles and chemically cross-linked biocompatible polymers are physically mixed
[0097] (1) Manufacture of extracellular matrix derived from human adipose tissue
[0098] Fat is removed by pulverizing human adipose tissue with a pulverizer. In order to remove undesorbed fat, a 16-hour degreasing process was performed using 40% to 60% isopropanol and 40% to 60% hexane. Cells were removed by treating fat-depleted tissue with 0.1 N sodium hydroxide (NaOH).
[0099] In order to clean and remove the extracellular matrix of fat and cells, centrifuge at 8000rpm for 10 minutes to remove the supernatant, and repeat the washing process 5 to 10 times. The scaffold is freeze-dried so that the moisture content of the human adipose tissue-derived extracellular matrix is less than 10%, preferably 1% to 8%.
[0100] The human adipose tissue-derived extracellular matrix that has been freeze...
experiment example 1
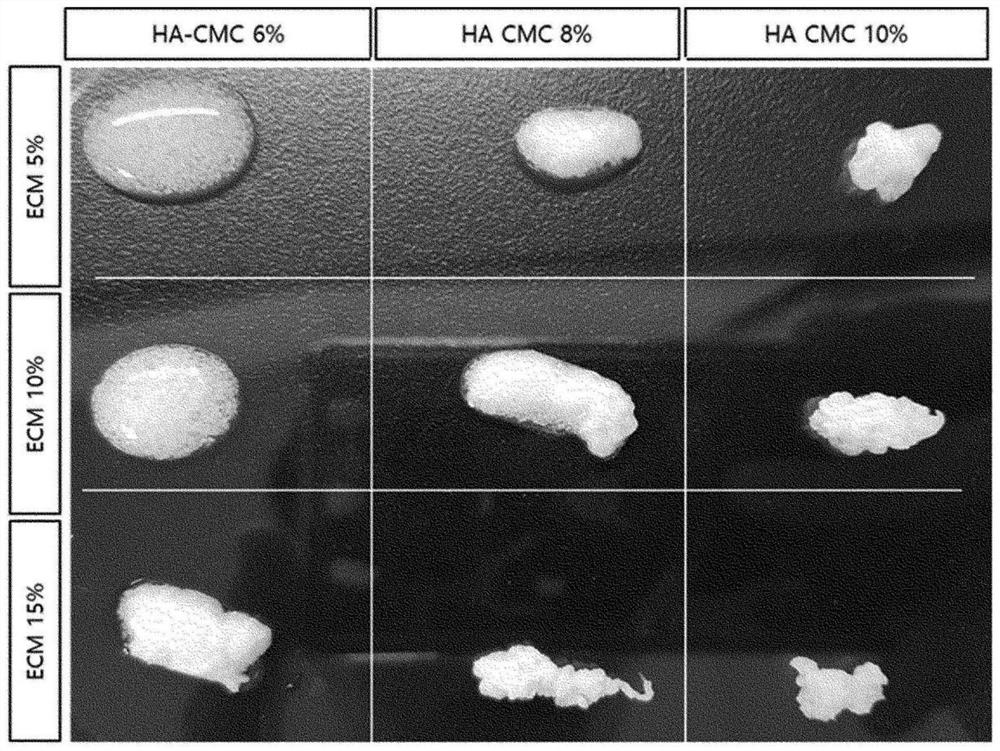
[0108] Experimental example 1. Verification of dosage form maintenance ability when medical composition is sterilized by radiation
[0109] (1) method
[0110] The dosage form maintenance ability of the medical composition produced in Example 1 was verified.
[0111] After preparing the samples with the contents in Table 1 below (remainder: sterilized physiological saline), they were sterilized with 25 kGy gamma rays. The physical properties by the content ratio of each component are confirmed at the time of gamma sterilization.
[0112] 【Table 1】
[0113]
[0114]
[0115] On the one hand, the pressing force on each sample was measured. Regarding the extrusion force, the maximum load value N is measured by using a universal testing machine, that is, the sleeve (cannula) is fixed to the syringe with the contents, and the syringe is pressed at a test speed of 12mm / min, so that the syringe The maximum load value N when the contents in the casing are discharged to the o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| elastic modulus | aaaaa | aaaaa |
| complex viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com