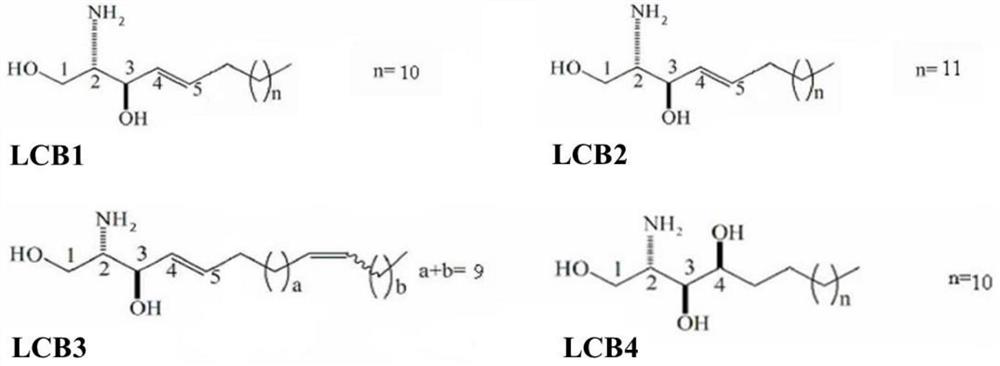
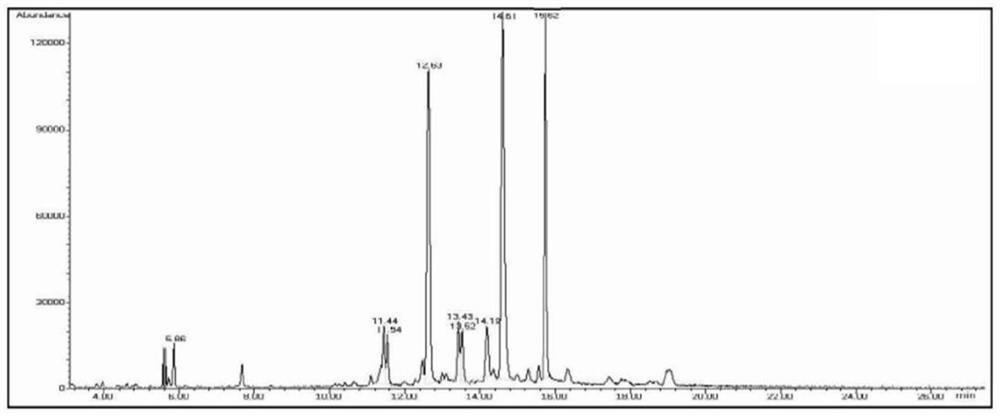
Application of sea cucumber long-chain alkali and derivatives thereof in preparation of products for regulating PPAR-gamma
A derivative and long-chain technology, which is applied in the application field of sea cucumber long-chain base and its derivatives in the preparation and regulation of PPAR-γ products, and achieves the effect of broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: PPAR-γ transactive test
[0050] The use of the long chain base (LCB) and / or their pharmaceutically acceptable salts of the present invention as the PPAR-gamma modifier in PPAR-γ-related diseases will be described by the conventional experiments described below.
[0051] The activation of the PPAR-γ receptor causes gene expression by an agonist (activator) in the human cervical cancer cell line (HELA cell line), and the luciferase will be emitted in the presence of a substrate. In the presence of a reference agonist, the regulation of the PPAR-γ receptor is measured by quantifying fluorescence generated after the cell culture. The ligand replaces the agonist from its site. The measurement of the activity is carried out by measuring the amount of light produced. This method of determining the compound of the invention is possible to determine the regulatory activity of the compound of the invention by measuring the molecules to be tested. Since this value can fl...
Embodiment 2
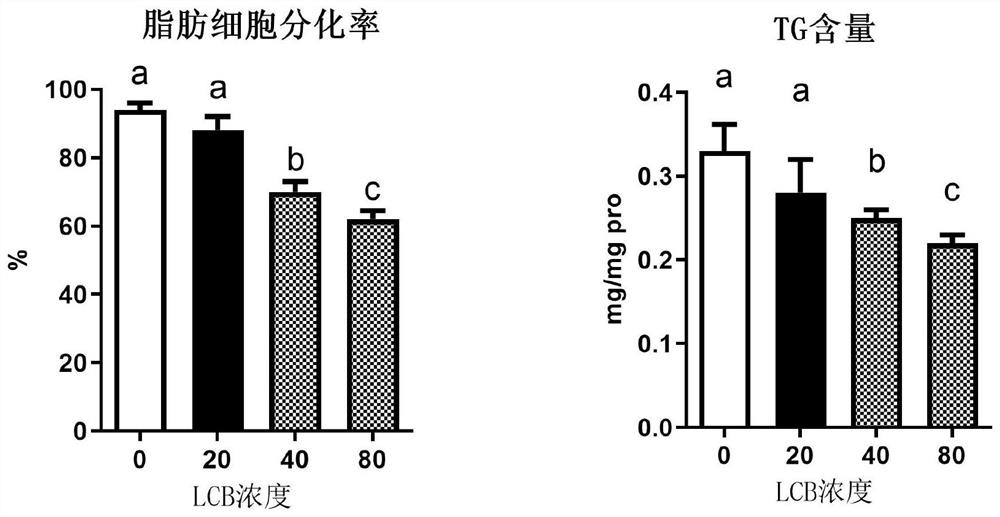
[0052] Example 2: Evaluation of the compound of the invention in a 3T3-L1 pre-fat cell model
[0053] The PPAR-γ regulation activity of the compound of the present invention is determined by measuring the mechanism of the long chain alkali 1 (LCB1, D17: 1) on the energic differentiation of the forekin cells.
[0054] 3T3-L1 cells were cultured in 5% CO2, 37 ° C incubator with a DMEM containing 10% FBS. Cells were converged to 90% or so. The concentration of the cells was adjusted and added to a 24-well plate, so that the number of cells per well was 2 × 104. After the cells were full and contacted inhibition of 48h, they were exchanged to contain FBS (10%), 3-isobutyl-1-methyl xanthine (0.5m mol / L), dexamethasone (1 μmol / L), insulin ( Continue in a fully medium medium in 10 μg / mL. After 48 h, a high sugar DMEM medium containing insulin (10 μg / mL) and FBS (10%). After each 2D, the serum volume fraction is 10% high sugar DMEM complete culture solution. A culture solution con...
Embodiment 3
[0055] Example 3: Evaluation of the compounds of the invention in a dietary induction obesity mouse model
[0056] The PPAR-γ regulation activity of the compound of the present invention is determined by measuring the regulation of fatty mice, blood fat, blood glucose, etc. of obese mice by measuring lepid base 1 (LCB1, D17: 1).
[0057] C57BL / 6 mice were divided into 4 groups: a low fat control group (LOW FAT, LF), high-fat obese group (HIGHFAT, HF), and high fat addition of 0.01% long chain base group (LCB). In addition to the low fat group, feed high-fat feed (22.5% (w / w) fat, 40% (w / w) sucrose), oral glucose tolerance experiment (OGTT) was carried out after 4 weeks. After another week, the mice were sacrificed and serum Tg and insulin were determined. The results show that LCB significantly inhibits high-fat mice oral glucose concentration and reduces blood insulin content, and has significant improved effect on insulin sensitivity and insulin resistance. Figure 4 A and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com