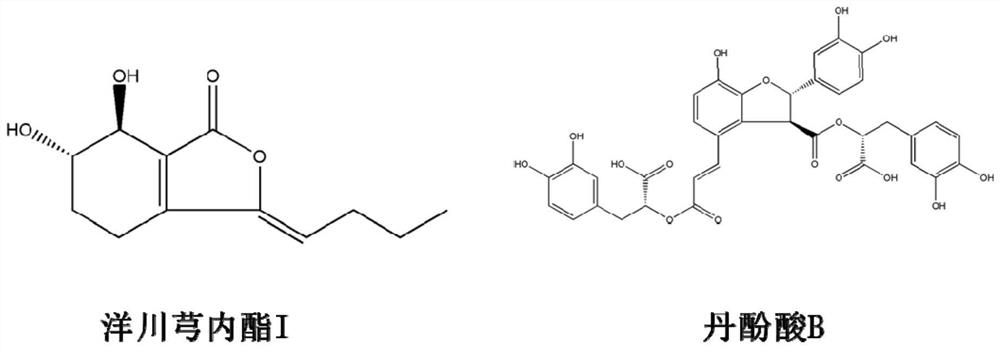
Skyunolide I compound and application thereof in treating myocardial hypertrophy disease
A technology of citrolactone and myocardial hypertrophy, which is applied in the field of medicine, can solve the problem of myocardial hypertrophy without citrolactone I, and achieve the effect of inhibiting myocardial hypertrophy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. Experimental animals and reagents
[0039] Neonatal SD rats (suckling mice) (1-3 days), male or female, Shanghai Slack Experimental Animal Co., Ltd., the experimental animal license number is SCXK (Shanghai) 2017-0005. The reagents and consumables are listed in Table 1.
[0040] Table 1
[0041]
[0042]
[0043] 2. Reagent preparation method
[0044] D-Hank's balanced salt solution: NaCl: 8g, KCl: 0.4g, NaCl 2 HPO 4 12H 2 O: 0.135g, KH 2 PO 4 : 0.06g, NaHCO 3 : 0.35g, dissolved in 1000mL pure water, adjusted to pH 7.0-7.2, tied with kraft paper, sterilized under high temperature and high pressure, and stored at 4°C after cooling.
[0045] DMEM / F12 culture medium: Dissolve 10.6g DMEM / F12 powder in 1000mL 15-20℃ pure water, stir with a magnetic stirrer for 30min, add 1.18g NaHCO 3 Until it is completely dissolved, adjust the pH to 7.0-7.2, filter through a 0.22 μm microporous membrane, and store at 4°C.
[0046] Fetal bovine serum (standard): 56°C, 30m...
Embodiment 2
[0081] 1. Experimental animals and reagents are the same as in Example 1.
[0082] 2. The culture process of neonatal rat cardiomyocytes is the same as in Example 1.
[0083] 3. Grouping and administration
[0084] Cells were cultured for 48 hours and replaced with serum-free medium, starved for 12 hours, modeling and drug addition were carried out at the same time, and divided into 9 groups, that is, the normal control group (Control): the cells were not treated, serum-free medium was added, and the high-glucose and high-insulin group (Model, HGI for short): High glucose (25.5mmol / L)+high insulin (0.1μmol / L) medium, HGI+salvianolic acid B group: HGI+80μmol / L, HGI+ ligustalactone I(A) group : HGI+100μmol / L, HGI+ Ligusticolide I (B) group: HGI+50μmol / L, HGI+ Ligusticolide I (C) group: HGI+20μmol / L, Compatibility Group A (HGI+salvianolic acid B 80μmol / L+ chuanxionglide I 100μmol / L), compatibility group B (HGI+ salvianolic acid B 80μmol / L+ chuanxionglide I 50μmol / L), compatibil...
Embodiment 3
[0104] 1. Experimental animals and reagents
[0105] Healthy 8-week-old C57BL / 6 male mice, weighing 20g±5g, SPF grade. Purchased from Shanghai Slack Experimental Animal Co., Ltd., the experimental animal license number is SCXK (Shanghai) 2017-0005. After one week of adaptive feeding, the animals were randomly divided into groups and the formal experiment began. The reagents and consumables are listed in Table 7.
[0106] Table 7
[0107]
[0108]
[0109] 2. Establishment, administration and sampling of ISO-induced myocardial hypertrophy model in mice
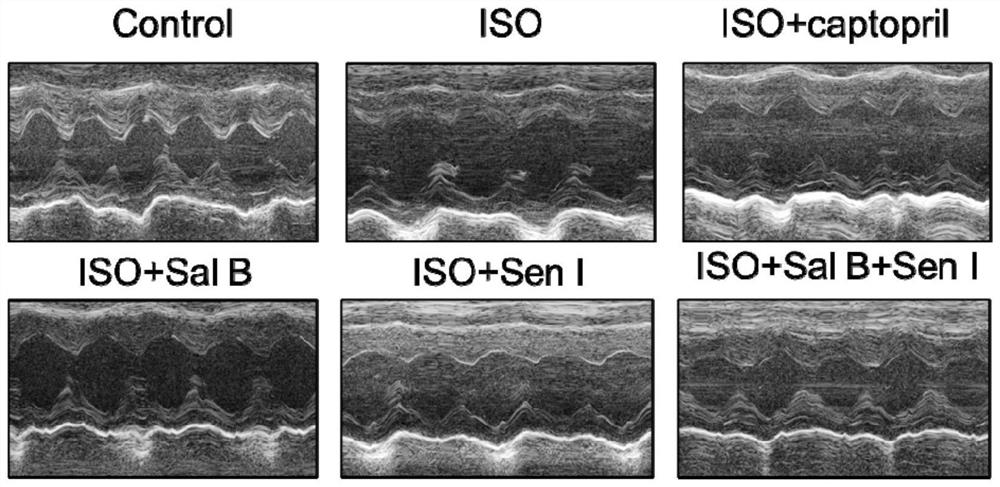
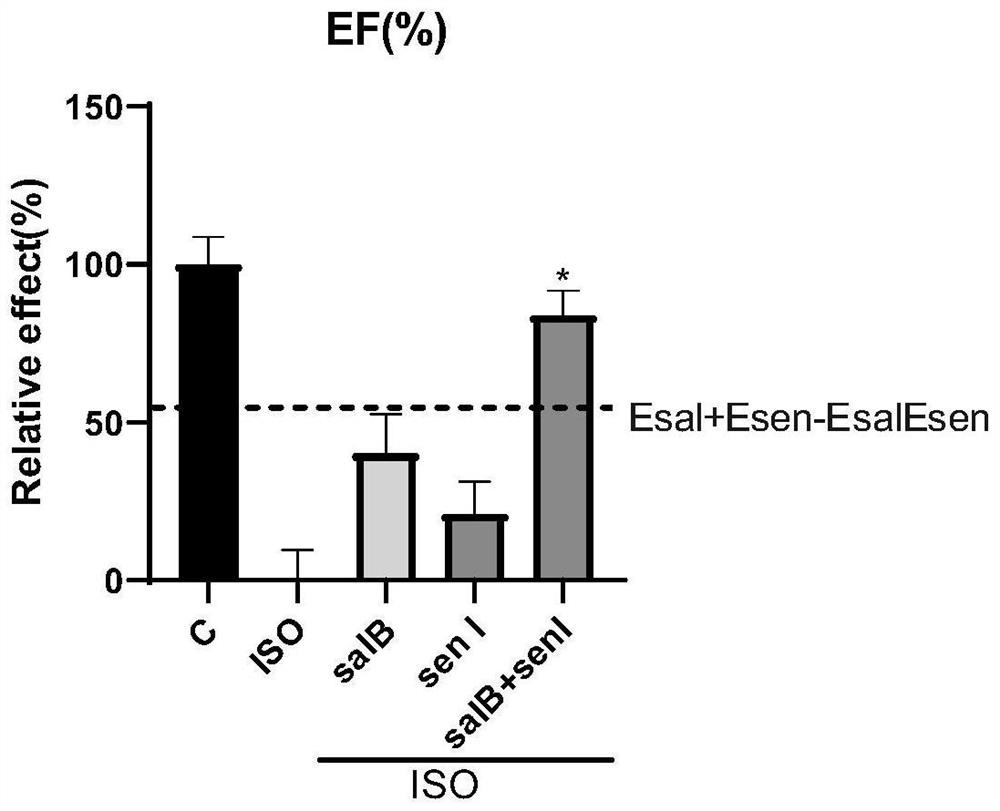
[0110] Male C57BL / 6 mice were randomly divided into 6 groups according to body weight, namely blank control group (Control), ISO group (Model), ISO+salvianolic acid B group, ISO+salvianolic acid I group, ISO+salvianolic acid B+salvianolic acid group Chuanxionglide I (compatibility) group, ISO+captopril group (positive drug), a total of 12 rats in each group, using the gradient modeling method, subcutaneous injection o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com