Rhamnolipid derivative as well as preparation method and application thereof
A rhamnolipid and double rhamnolipid technology, which is applied in the field of daily chemicals, can solve problems such as the compatibility between rhamnolipid and silicone oil, and achieve the expansion of types and application ranges, simple process, Effect of improving skin feel
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The preparation of embodiment 1 monorhamnolipid derivative
[0054] References prepared a mixture with dirhamnolipid as the main component, and confirmed by HPLC that the content of monorhamnolipid was 1.51%, and that of dirhamnolipid was 80.8%. Select this mixture, add 200ppm rhamnosidase, react overnight at 50°C, analyze by HPLC until the product is completely converted into monosaccharide, heat to 80°C to inactivate the enzyme, freeze-dry to obtain monorhamnolipid product.
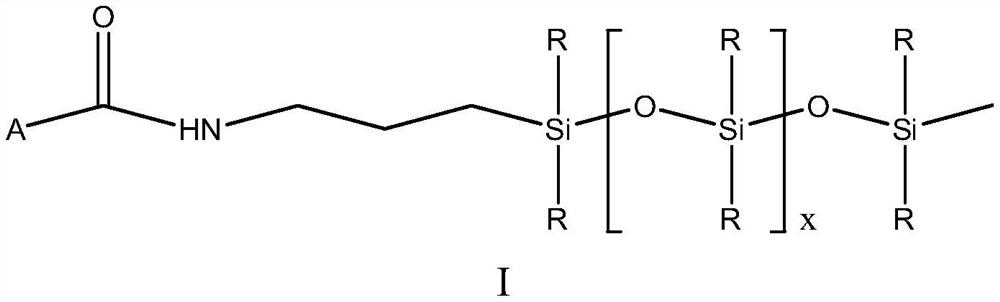
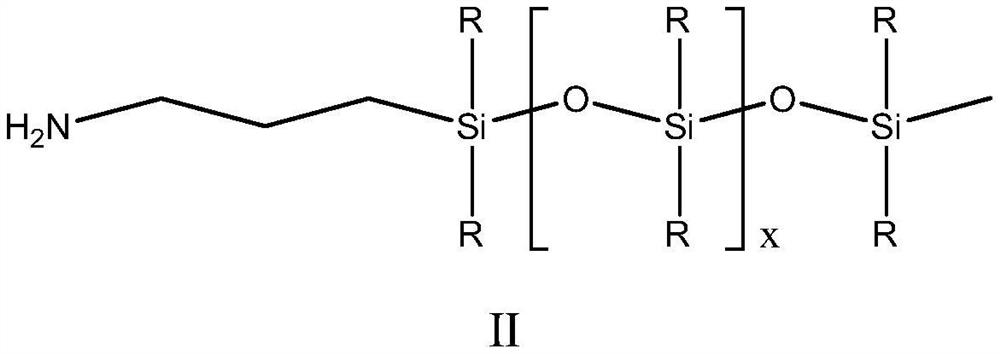
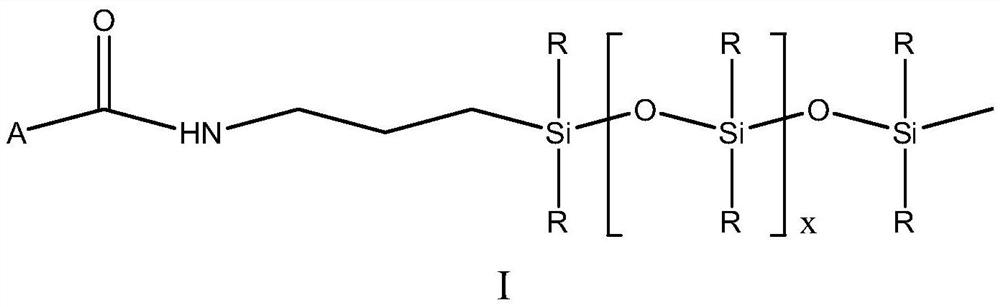
[0055] 45g of the prepared monorhamnolipid and 20.6g of dicyclohexylcarbodiimide were dissolved in 225g of tetrahydrofuran at 50°C. After it was completely dissolved, 9.9g of N-hydroxysuccinimide was added, and then 141.7g of Amino silicone oil (the average segment length is about 10, the molar ratio of monorhamnolipid and amino silicone oil is about 1:1.5) is slowly added to the system through a peristaltic pump, the addition speed is 1g / min, and the reaction temperature is controlled at 40- 50...
Embodiment 2
[0057] The preparation of embodiment 2 monorhamnolipid derivatives
[0058] Monorhamnolipid was prepared according to the method of Example 1.
[0059] 45g of the prepared monorhamnolipid and 20.6g of dicyclohexylcarbodiimide were dissolved in 225g of tetrahydrofuran at 50°C. After it was completely dissolved, 9.9g of N-hydroxysuccinimide was added, and then 157.8g of Amino silicone oil (the average segment length is about 15, the molar ratio of monorhamnolipid and amino silicone oil is about 1:1.5) is slowly added to the system through a peristaltic pump at a rate of 1g / min, and the reaction temperature is controlled at 50- 60°C, after the reaction was over, a crude product was obtained. According to the method of Example 1, the crude product was separated to obtain monorhamnolipid derivative 2.
Embodiment 3
[0060] The preparation of embodiment 3 rhamnolipid derivatives
[0061] References A mixture with dirhamnolipid as the main component was prepared, and the structure was confirmed by HPLC. The content of monorhamnolipid was 1.51%, and the content of dirhamnolipid was 80.8%.
[0062] Dissolve 65g of the prepared bisrhamnolipid and 20.6g of dicyclohexylcarbodiimide in 325g of tetrahydrofuran at 50°C. After it is completely dissolved, add 9.9g of N-hydroxysuccinimide, and then 94.5g of Amino silicone oil (the average segment length is about 10, the molar ratio of dirhamnolipid to amino silicone oil is about 1:1.2) is slowly added to the system through a peristaltic pump, the addition speed is 1g / min, and the reaction temperature is 50-60 °C, after the reaction was over, the crude product was obtained. According to the method of Example 1, the crude product was separated to obtain rhamnolipid derivative 3.
[0063] h 1 NMR (400Hz, deuterated DMSO, TMS as internal standard): δ: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com