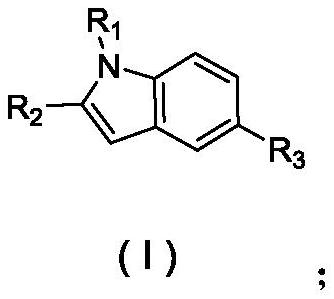
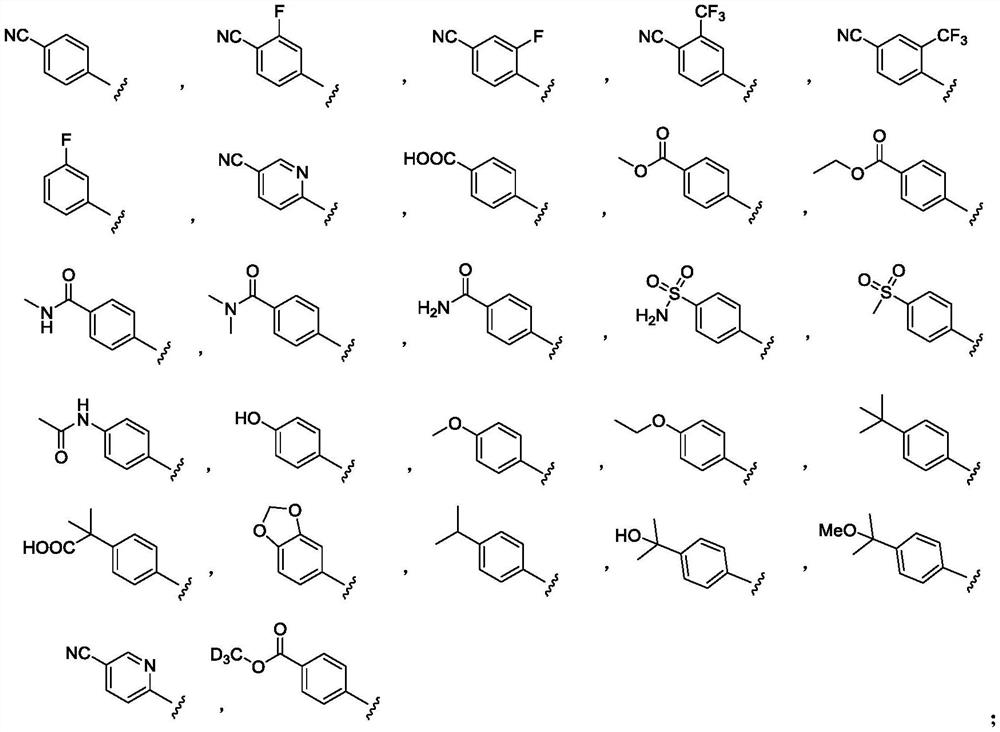
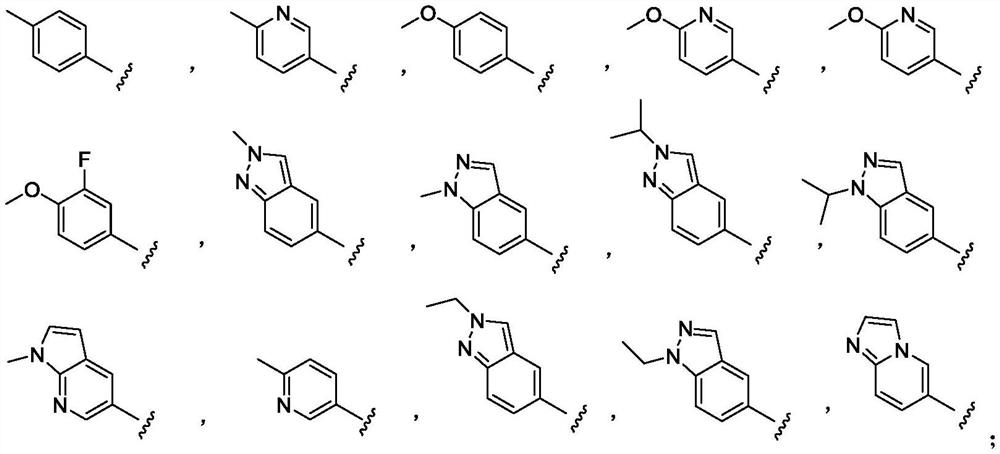
Indole derivative as well as synthesis method and application thereof
A synthesis method and technology of application, applied in the field of chemistry, to achieve the effect of inhibiting proliferation and inhibiting the proliferation of cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: 4-(5-(4-aminopiperidin-1-yl)-2-(3-fluoro-4-methoxyphenyl)-1H-indol-1-yl)benzonitrile
[0070]
[0071] Step 1: Synthesis of 4-ethynyl-2-fluoro-1-methoxybenzene
[0072]
[0073] Add 3-fluoro-4-methoxybenzaldehyde (1.0eq), MeOH, (1-diazo-2-oxo-propanol)-phosphonic acid dimethyl ester (2.2eq), K 2 CO 3 (2.2eq), the addition was completed, and the reaction was stirred overnight at room temperature. After the reaction was complete, the solvent was spin-dried and purified by column chromatography (by volume, PE:EA=10:1, PE refers to petroleum ether, EA refers to ethyl acetate, and the following meanings are the same), and the title The compound is light yellow oily liquid.
[0074] Step 2: Synthesis of 4-bromo-2-((4-methoxy-3-methylphenyl)ethynyl)aniline
[0075]
[0076]Add 4-ethynyl-2-fluoro-1-methoxybenzene (1.2 eq), 4-bromo-2-iodoaniline (1.0 eq), DMA (dimethylacetamide, CAS accession number: 127-19-5), Pd(PPh 3 ) 2 Cl 2 (0.2eq), CuI(0.2eq), E...
Embodiment 2
[0087] Example 2: 4-(2-(3-fluoro-4-methoxyphenyl)-5-(4-(methylamino)piperidin-1-yl)-1H-indol-1-yl) Benzonitrile (i.e. compound I-2)
[0088]
[0089] Step 1: Synthesis of (1-(1-(4-cyanophenyl)-2-(3-fluoro-4-methoxyphenyl)-1H-indol-5-yl)piperidin-4-yl )(methyl)carbamate tert-butyl ester
[0090]
[0091] Add 4-(5-bromo-2-(3-fluoro-4-methoxyphenyl)-1H-indol-1-yl)benzonitrile (1.0 eq), tert-butylmethyl (piperidine -4-yl) carbamate (1.0eq), Pd 2 (dba) 3 (0.11eq), cesium carbonate (2.0eq), X-phos (0.22eq), dioxane, under nitrogen protection, heated to 110°C to react, TLC to monitor the reaction progress. After the reaction was complete, the reaction solution was filtered with a membrane filter, the filtrate was spin-dried, and the residue was purified by column chromatography (PE:EA=3:2) to obtain the title compound as a yellow solid. MS-ES: (ESI, pos.ion) m / z: 499.30 [M+H]+.
[0092] Step 2: 4-(2-(3-fluoro-4-methoxyphenyl)-5-(4-(methylamino)piperidin-1-yl)-1H-indol-1-y...
Embodiment 3
[0095] Example 3: 4-(5-(4-aminopiperidin-1-yl)-2-(3-fluoro-4-methoxyphenyl)-1H-indol-1-yl)-2-hydroxy Benzonitrile
[0096]
[0097] Step 1: Synthesis of 5-bromo-2-cyanophenyl tert-butyl carbonate
[0098]
[0099] Add 4-bromo-2-hydroxybenzonitrile (1.0eq), THF (10V), DMAP (0.2eq), BOC anhydride (1.3eq, di-tert-butyl dicarbonate), TEA (1.5eq, tris ethanolamine), the addition was completed, the reaction was stirred at room temperature, and the reaction progress was monitored by TLC. After the reaction is complete, add ethyl acetate (10V) to the reaction flask, then use 1N dilute hydrochloric acid to adjust the pH of the system to 3, separate the liquids, wash the organic phase with saturated brine, dry over anhydrous sodium sulfate, filter and spin to dry the solvent, and the residual The product was purified by column (PE:E A=3:1) to obtain the title compound as a colorless oil.
[0100] Step 2: Synthesis of 5-(5-bromo-2-(3-fluoro-4-methoxyphenyl)-1H-indol-1-yl)-2-cyan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com