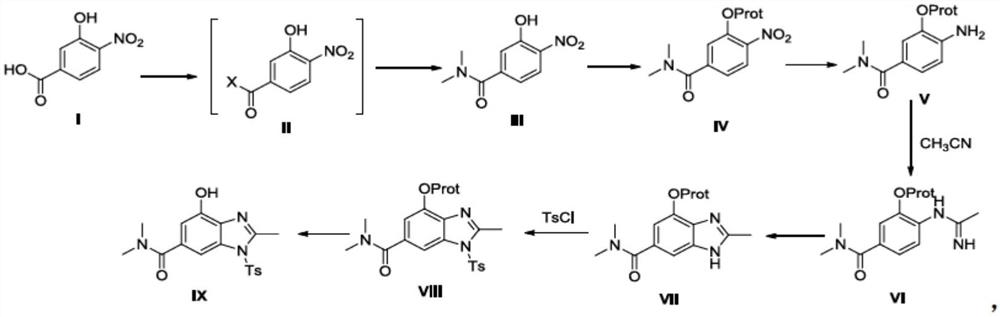
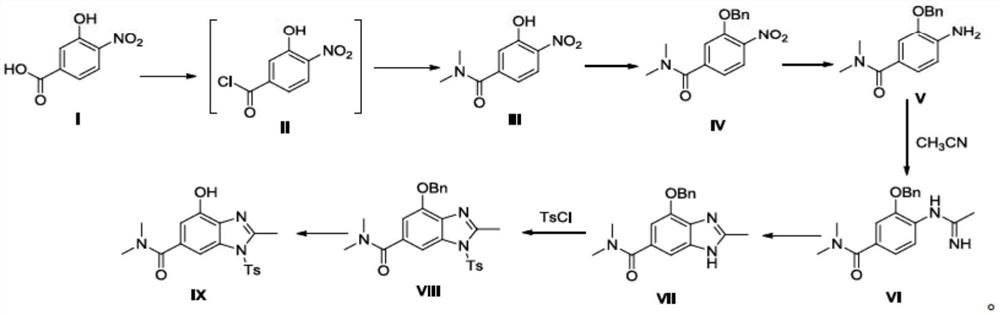
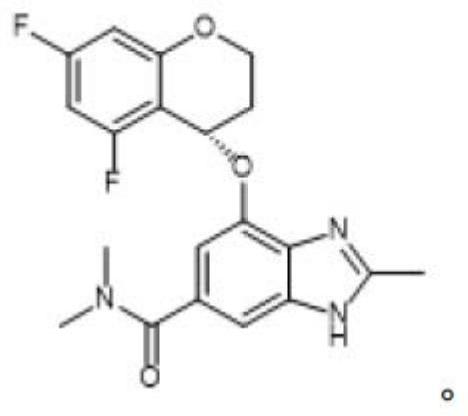
Preparation method of terglazan intermediate
A Teglazan and Asana technology, which is applied in the field of preparation of Teglazan intermediates, can solve the problems of difficulty in realizing industrialized production, dangerous operation, and complicated post-processing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1: the synthesis of compound III'
[0064]
[0065] Put 3-hydroxy-4-nitrobenzoic acid (183g, 1.0mol), dry acetonitrile (1.0L), DMF (3.7g, 2.0mol) into a 5L three-necked flask, start mechanical stirring, slowly heat up to 70°C, and then add Thionyl chloride (238.0 g, 2.0 mol) was slowly added dropwise, and the drop was completed within 0.5-1.0 hours, and the reaction was continued for 2 hours. Then the temperature was lowered to 0° C., dimethylamine hydrochloride (163.0 g, 2.0 mol) was dropped into the reaction solution, stirring was continued for 0.5 hour, triethylamine (405.0 g, 4.0 mol) was slowly added dropwise, and the dropwise was completed within 1.0 hour. The temperature was raised to room temperature, and the reaction was continued for 0.5 hours. Evaporate the solvent under reduced pressure at 40-50°C, add 300mL of water, adjust the pH value to 3-4 with 2N dilute hydrochloric acid, extract twice with dichloromethane (500ml x 3), discard the aqueou...
Embodiment 2
[0066] Embodiment 2: the synthesis of compound IV'
[0067]
[0068] Put 3-hydroxy-N, N-dimethyl-4-nitrobenzamide (168.2g, 0.80mol), acetone (800mL), potassium carbonate (221.1g, 0.016mol) into a 2.0L three-necked flask, start mechanical stirring, and keep warm at room temperature for 10 minutes , and then slowly drop benzyl bromide (136.8 g, 0.80 mol) into the reaction solution, and drop it within 0.5-1.0 hours. After the reaction is complete, evaporate the solvent to dryness under reduced pressure at 40-50°C, add 500mL of water, extract twice with isopropyl acetate (500ml x 2), discard the aqueous phase, combine the organic phases, and concentrate to dryness at 35-40°C under reduced pressure to obtain Yellow viscous substance (233.3 g, 0.78 mol), namely the target compound IV', yield 97.2%. 1 H NMR (400MHz, CDCl 3 )δ2.96(s, 3H), 3.09(s, 3H), 5.25(s, 2H), 7.04(d, 2H, J=8.4Hz), 7.16(s, 1H), 7.32~7.44(m, 5H ), 7.87 (d, 1H, J = 8.0 Hz). MS(ESI): m / z 301.1132[M+H] + .
Embodiment 3
[0069] Embodiment 3: the synthesis of compound V'
[0070]
[0071] Put 3-(benzyloxy)-N, N-dimethyl-4-nitrobenzamide (180.2g, 0.6mol), dichloromethane (800ml), acetic acid (180.2g, 1.8mol) into a 1.0L three-necked flask, start mechanical stirring, and stir at room temperature After 10 minutes, Zn powder (78.5 g, 1.2 mol) was added in batches in four batches, 19.6 g in each batch, with an interval of 1.5 h. The progress of the reaction was tracked by HPLC, and the reaction was complete after 7 hours. Filter, wash the filter cake with 200mL dichloromethane, add 500mL water to the filtrate, stir and separate layers, extract the water phase twice with dichloromethane (500ml x 2), discard the water phase, combine the organic phases, and reduce Concentrate to dryness under reduced pressure to obtain a yellow viscous substance (150.2 g, 0.56 mol), which is the target compound V', with a yield of 92.5%. 1 H NMR (400MHz, CDCl 3 )δ3.01(s, 6H), 4.02(s, 2H), 5.09(s, 2H), 6.68(d, 1H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com