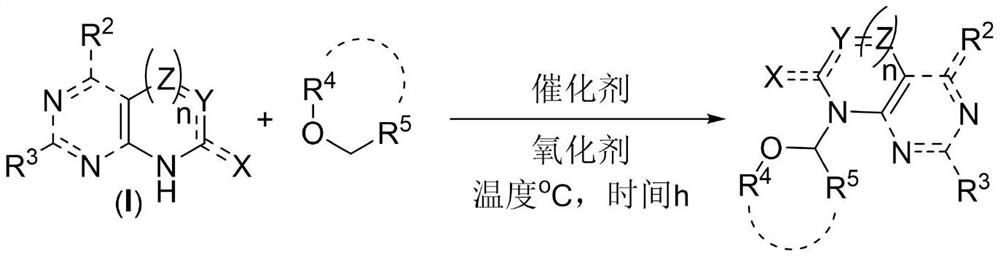
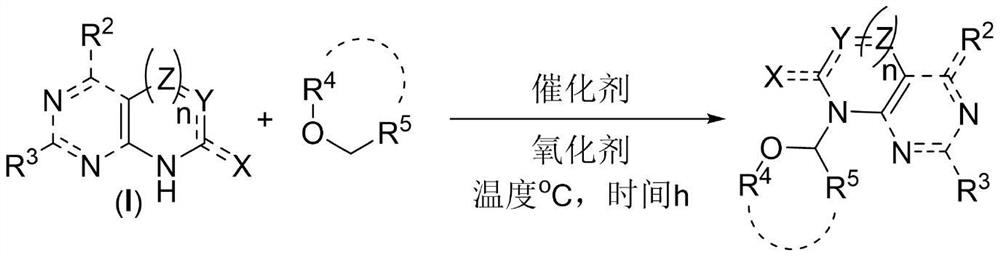
One-step synthesis method of 2 '3'-dideoxynucleoside
A technology of deoxynucleoside and synthesis method, applied in the field of organic chemical industry, can solve the problems of long steps, low efficiency, etc., and achieve the effects of short time, mild reaction conditions and wide selection of ligands
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
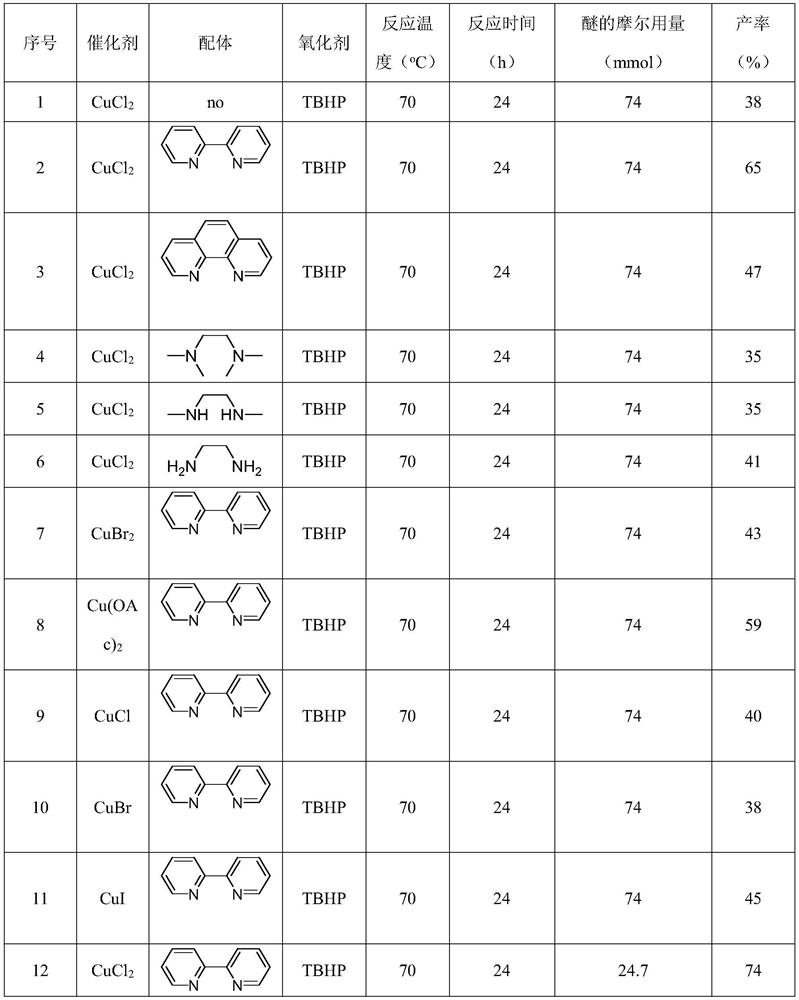
[0048] Put 1mmol 2,6-dichloropurine, 24.7mmol tetrahydrofuran, 5mmol tert-butanol peroxide, 0.1mmol 2,2'-bipyridine, 0.1mmol copper chloride in the reactor, and stir at 70°C for 24 hours. After the reaction, excess tetrahydrofuran was distilled off by rotary distillation, and 191 mg of the product was obtained through silica gel column chromatography. The ratio of the theoretical yield to 258 mg was calculated to give a reaction yield of 74%. The reaction equation is as follows:
[0049]
[0050] The product passed the nuclear magnetic resonance test, and the measured data were: 1 H NMR (400MHz, CDCl 3 ,TMS)δ8.23(s,1H),6.32(t,J=4.2Hz,1H),4.21(dd,J=14.4Hz,7.8Hz,1H),4.10(dd,J=16.0Hz,7.6 Hz,1H),2.56(m,2H),2.17(m,2H); 13 C NMR (100MHz, CDCl 3 )δ152.7, 152.1, 151.6, 144.1, 131.4, 86.7, 70.1, 32.7, 24.2. The product has been tested by high-resolution mass spectrometry, and the test data obtained is: HRMS (EI) Calcd forC 9 h 8 Cl 2 N 4 O:[M] + 258.0075; Found, 258.0079. ...
Embodiment 2
[0052] 1mmol 6-chloropurine, 24.7mmol tetrahydrofuran, 5mmol tert-butanol peroxide, 0.1mmol 2,2'-bipyridine, 0.1mmol copper chloride were placed in the reactor, and stirred at 70°C for 24 hours. After the reaction was completed, excessive THF was evaporated by rotary distillation, and 146 mg of the product was obtained through silica gel column chromatography. The ratio of the theoretical yield to 225 mg was calculated to give a reaction yield of 65%. The reaction equation is as follows:
[0053]
[0054] The product was tested by nuclear magnetic resonance, and the measured data were: 1H NMR (400MHz, CDCl3, TMS) δ8.75(s, 1H), 8.24(s, 1H), 6.36(dd, J=5.6Hz, 2.6Hz, 1H ), 4.32(dd, J=10.8Hz, 6.6Hz, 1H), 4.11 (dd, J=10.8Hz, 7.8Hz, 1H), 2.59(m, 2H), 2.18(m, 2H); 13C NMR (100MHz , CDCl3) δ 151.8, 151.0, 150.9, 143.4, 132.4, 86.6, 69.9, 32.5, 24.2. The product was tested by high-resolution mass spectrometry, and the test data obtained was: HRMS (ESI) Calcd for C 9 h 9 ClN 4 O:...
Embodiment 3
[0056] 1 mmol of bis-Boc-adenine, 24.7 mmol of tetrahydrofuran, 5 mmol of tert-butanol peroxide, 0.1 mmol of 2,2'-bipyridine, and 0.1 mmol of copper chloride were placed in a reactor and stirred at 70°C for 24 hours. After the reaction was completed, excess tetrahydrofuran was distilled off, and 207 mg of the product was obtained through silica gel column chromatography. The ratio of the theoretical yield to 406 mg was calculated to give a reaction yield of 51%. The reaction equation is as follows:
[0057]
[0058] The product passed the nuclear magnetic resonance test, and the measured data were: 1 H NMR (400MHz, d 6 -DMSO)δ8.85(s,1H),8.75(s,1H),6.41(dd,J=6.4Hz,3.6Hz,1H),4.16(dd,J=14.8Hz,7.6Hz,1H),3.94 (dd, J=14.4Hz, 7.0Hz, 1H), 2.44-2.55(m, 2H), 2.23(m, 1H), 2.06(m, 1H), 1.38(s, 18H); 13 C NMR (100MHz, CDCl 3)δ152.5, 151.9, 150.5, 150.2, 142.9, 129.5, 86.2, 83.7, 69.8, 32.4, 27.8, 24.3. The product has been tested by high-resolution mass spectrometry, and the test da...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com