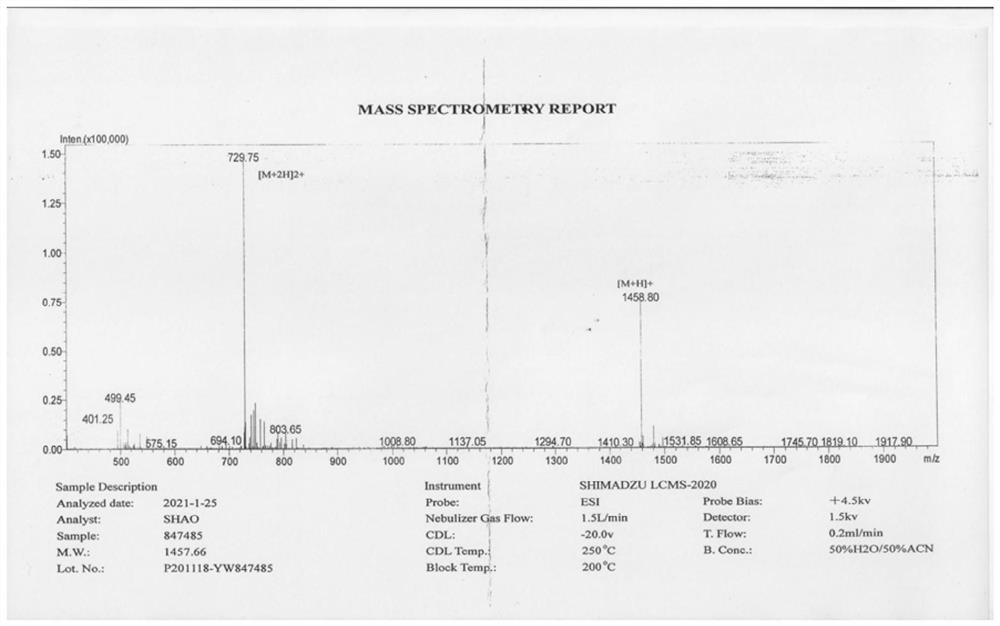
68Ga-NODAGA-cyclic polypeptide FG01 targeting EphA2 receptor as well as preparation method and application of 68Ga-NODAGA-cyclic polypeptide FG01
A 68ga-nodaga-, cyclic polypeptide technology, applied in the field of biomedicine, can solve the problems of not being able to know the treatment effect in time, not suitable for clinical examination of patients with hyperglycemia, and not being able to see changes in blood vessels and lymphatic vessels, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] A targeting EphA2 receptor 68 The preparation method of Ga-NODAGA-cyclic polypeptide FG01, the specific steps are as follows:
[0055] (1) Adopt the Fmoc scheme, select Wang resin, connect the serine with the tBu protecting group at the carboxyl end of the cyclic polypeptide to the Wang resin, remove the Fmoc protecting group, and use the serine bound on the Wang resin as the starting point for synthesis According to the amino acid sequence MMPVSDPYK from the carboxy-terminus to the amino-terminus, the condensation reaction is carried out successively to form peptide bonds, in which amino acids S, D, Y, and K are all amino acids with protective groups, which are removed from Fmoc protection, condensed to form peptide bonds, and repeated This step is repeated until all the sequences are coupled completely, and the polypeptide is cleaved from the Wang resin and removed from Fmoc protection to obtain a polypeptide sequence with protective groups for amino acids S, D, Y, an...
Embodiment 2
[0071] Example 2 targeting the EphA2 receptor 68 In Vitro Radiochemical Properties of Ga-NODAGA-Cyclic Polypeptide FG01
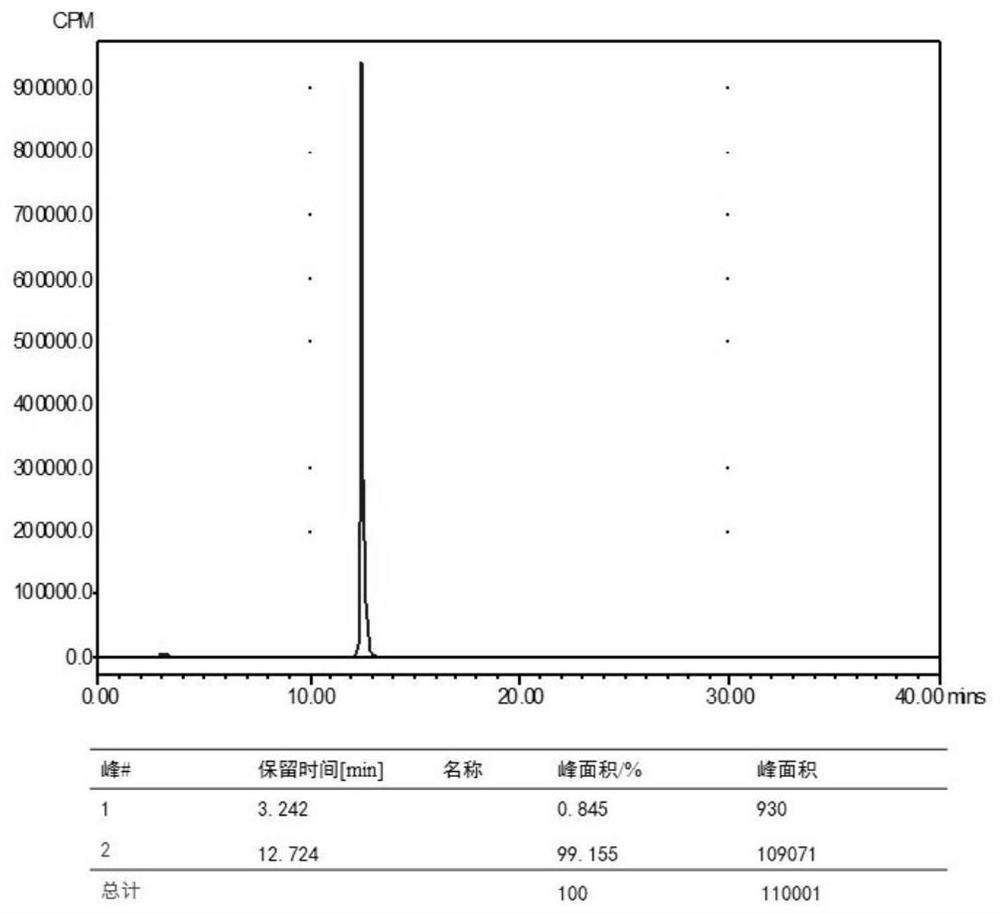
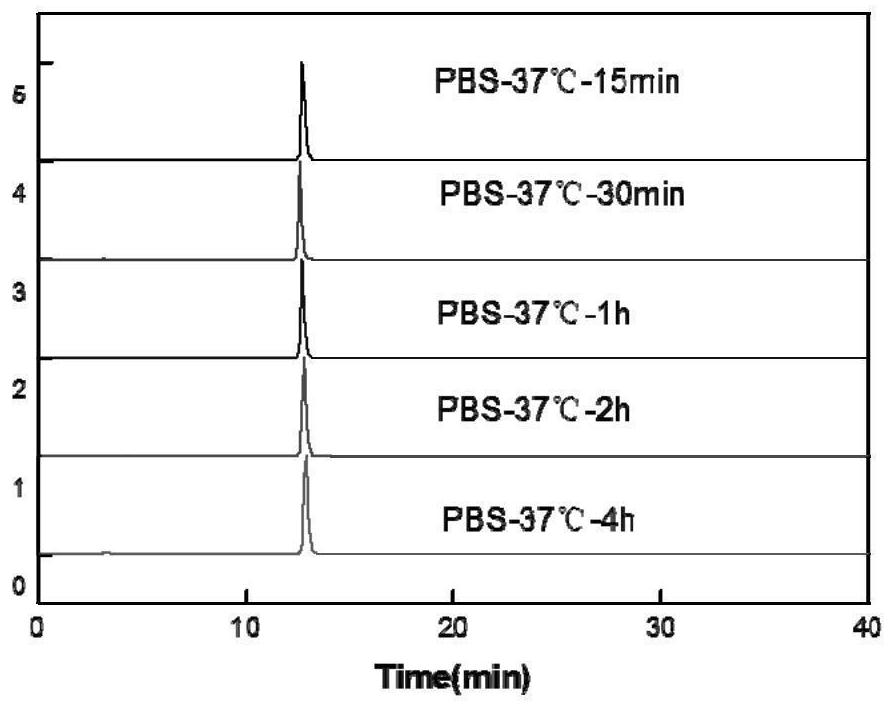
[0072] In vitro stability: 20 μL of the prepared Example 1 68 Ga-NODAGA-cyclic polypeptide FG01 was added to 180 μL of PBS buffer with pH=7.4, incubated at 37°C for 15min, 30min, 1h, 2h, and 4h, and then 20μL of the mixture was taken out and injected into radioactive HPLC for detection. The results are as follows: Figure 4 shown.
[0073] Depend on Figure 4 known in vitro 68 The radiochemical purity of Ga-NODAGA-cyclic polypeptide FG01 has no significant change and is above 95%, and has good in vitro stability within 4 hours.
[0074] Hydrophilic and lipophilic: prepared in Example 1 of 0.15MBq 68 Ga-NODAGA-cyclic polypeptide FG01 was diluted to 500 μL with HEPES buffer at pH=7.4, and then 500 μL of n-octanol was added and shaken vigorously. Take an equal amount of liquid from each of the aqueous and organic phases to measure their radioactive count...
Embodiment 3
[0078] Example 3 Targeting the EphA2 receptor 68 Cell growth inhibition and cellular uptake of Ga-NODAGA-cyclic polypeptide FG01
[0079] Cell culture: in 5% CO 2 , non-small cell lung cancer cells (A549 and NCI-H1299) were cultured in RPMI 1640 medium containing 10% calf serum in a 37°C cell culture incubator.
[0080] MTT experiment: 10 μL of the cyclic polypeptide FG01 prepared in Example 1 with a concentration of 0.039, 0.195, 0.976, 4.88, 24.4, and 122 μM was added to non-small cell lung cancer cells (A549 and NCI-H1299) containing 90 μL of culture solution, In different time periods (5, 24, 48 and 72h), the effect of the cyclic polypeptide FG01 on cell growth was observed, and the results are shown in Figure 5, Image 6 shown.
[0081] Depend on Figure 5 with Image 6 It can be seen that the cyclic polypeptide FG01 has no significant effect on the growth of non-small cell lung cancer, indicating that the cyclic polypeptide FG01 has no cytotoxicity.
[0082] Cell u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com