Soluble NK-CAR fusion protein as well as preparation method and application of soluble NK-CAR fusion protein in drug for mediating immune cells to kill tumor cells
A technology of NK-CAR and fusion protein, which is applied in the direction of anti-tumor drugs, DNA/RNA fragments, drug combinations, etc., can solve the problems of enhanced cytotoxic activity, achieve the effects of prolonging metabolic half-life, facilitating protein purification, and saving production time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment one: the construction of pCMV5.1-MS-Ig recombinant plasmid
[0049] The nucleotide sequence of the extracellular region of MICA was queried from IPD-IMGT / HLA Database, the anti-CD20 single-chain antibody sequence was preserved in our laboratory, and the IgG Fc nucleotide sequence was queried from PubMed. Such as figure 1 As shown, the above sequences were spliced in sequence, and the recombinant sequences were synthesized by BGI. The synthesized sequence and the pCMV5.1 plasmid were subjected to double enzyme digestion at the same time, and the digested product was run on agarose gel, and the gel was cut to recover the digested recombinant sequence and the corresponding band of the empty plasmid vector, and then ligated with T4 ligase. The product was transformed into DH5α Escherichia coli competent cells, and the positive monoclonals were screened by smearing resistance plates, the recombinant plasmids were verified by enzyme digestion, and sent to the c...
Embodiment 2
[0052] Example 2: Expression of soluble NK-CAR MS-Ig
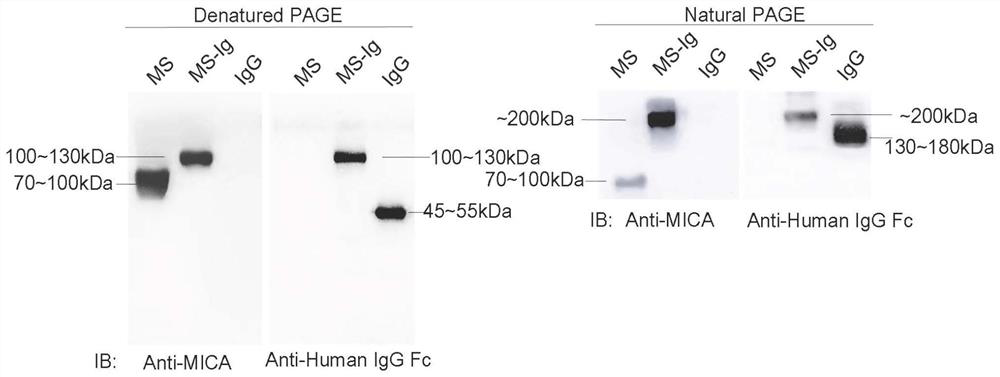
[0053] Recover and culture HEK293 suspension cells in large quantities, and extract a large amount of pCMV5.1-MS-Ig recombinant plasmid at the same time, mix PEI and plasmid at a volume ratio of 3:1, incubate at 25°C for 15 minutes, and transfect at a final concentration of 1 μg / ml plasmid , 7 days after transfection, the cells were centrifuged, and the supernatant was taken to verify the expression of recombinant protein by western blotting. The result is as figure 2 As shown, a disulfide bond is formed between the Fc ends of two monomers of the fusion protein, so that the protein is in a dimer state under natural conditions. After the protein is denatured, the disulfide bond is destroyed and the protein returns to a monomer. "Denatured PAGE" corresponds to the immunoblotting results of proteins denatured by heating and running 10% reducing gel, and "Natural PAGE" corresponds to the immunoblotting results of proteins no...
Embodiment 3
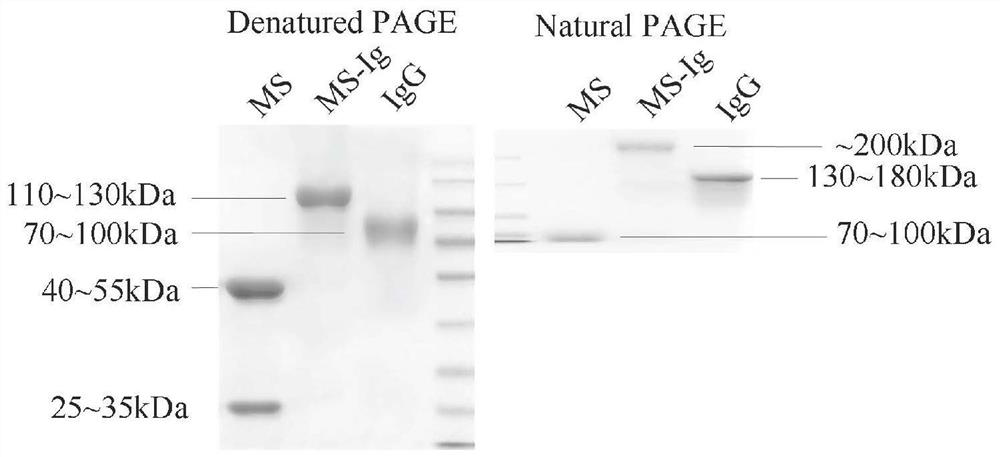
[0054] Example 3: Purification of soluble NK-CAR MS-Ig
[0055] Draw an appropriate amount of SPA microsphere packing into the protein purification column, first balance the column with the company's matching balance solution at a flow rate of 30ml / min, dilute the cell culture supernatant collected after the above expression with the balance solution at a ratio of 1:4, and place it on ice and pass the supernatant through the column at a flow rate of 2ml / min. After passing through the column, wash the column with a balance solution at a flow rate of 30ml / min. The flow rate of min was eluted, and the collection tube was used for collection. The collection tube was added with an appropriate amount of neutralizing solution in advance to restore the pH value of the collected protein eluate to 7.4. Concentrate the eluted protein solution with a protein ultrafiltration tube, replace the protein solvent with PBS, detect the concentration of the concentrated protein, and store it at -8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com