Rifapentine-containing pharmaceutical composition and preparation method thereof
A technology of rifapentine and rifapentine layer, which is applied in the field of pharmaceutical preparations, can solve the problems such as the impurity content cannot be reduced, and achieve the effect of improving safety and drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059]Embodiment 1 rifapentine crude drug
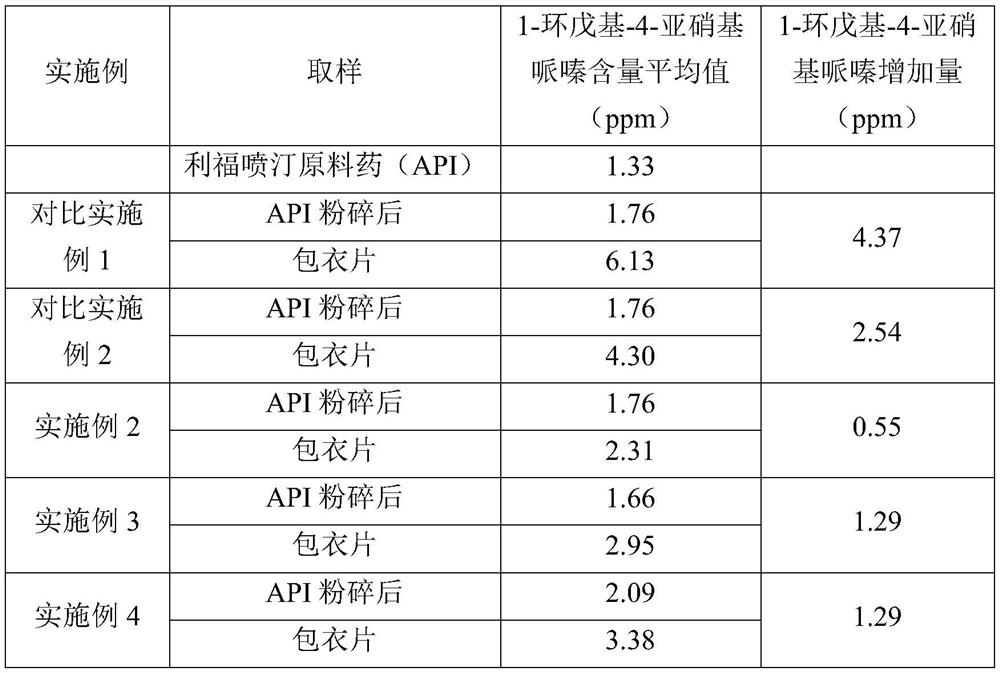
[0060] The method disclosed in the patent application CN111018886A was adopted, wherein the process of crystallization, recrystallization and purification in step 5 was repeated many times to obtain the raw material drug of rifapentine, which was tested for 1-cyclopentyl-4-nitrosopiper The content of oxazine was 1.33 ppm.
Embodiment 2
[0062] prescription
[0063] Rifapentine layer
[0064] Intragranular Weight (mg / tablet) Rifapentine 300.0 Microcrystalline Cellulose PH112 150 Sodium carboxymethyl starch 20 pregelatinized starch 30 Calcium stearate 3 Extragranular Microcrystalline Cellulose PH112 60 sodium ascorbate 10 Sodium carboxymethyl starch 26 Calcium stearate 4 total 603
[0065] Isoniazid layer
[0066] Isoniazid 300.0 Microcrystalline Cellulose PH102 150.0 Microcrystalline Cellulose KG802 40.0 Sodium carboxymethyl starch 20.0 Calcium stearate 0.25 total 510.25
[0067] Preparation process (dry granulation)
[0068] (1) Micronize the prescribed amount of rifapentine in advance, mix with microcrystalline cellulose PH112, sodium carboxymethyl starch, and pregelatinized starch for 10 minutes, and then add calcium stearate passed through a 60-mesh sieve and mix 3 minutes, wher...
Embodiment 3
[0076] prescription
[0077] Rifapentine layer
[0078] Intragranular Weight (mg / tablet) Rifapentine 300.0 Microcrystalline Cellulose PH102 145.5 Low-substituted hydroxypropyl cellulose LC21 10 pregelatinized starch 20 Sodium dodecyl sulfate 2.5 Calcium stearate 3 Extragranular Microcrystalline Cellulose KG802 40 sodium ascorbate 10 Sodium carboxymethyl starch 5 Calcium stearate 4 total 540
[0079] Isoniazid layer
[0080] Isoniazid 300.0 Microcrystalline Cellulose PH102 159.75 Microcrystalline Cellulose KG802 40.0 Sodium carboxymethyl starch 30.0 Low-substituted hydroxypropyl cellulose LC21 15.0 Calcium stearate 0.25 total 545
[0081] Preparation process (dry granulation)
[0082] According to the prescription in the table above, the rifapentine-containing composition was prepared by the preparation method of Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com