Novel hydroxychloroquine eutectic crystal, and preparation method, content determination method and application thereof
A hydroxychloroquine and crystallization technology, applied in the field of hydroxychloroquine new co-crystal and preparation thereof, can solve problems such as quantitative analysis of unfavorable hydroxychloroquine salt compounds, difficulties in industrial production and storage, and achieve low hygroscopicity and tabletability, Good linear relationship and less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] This embodiment has prepared a kind of new eutectic of hydroxychloroquine, and specific process is:
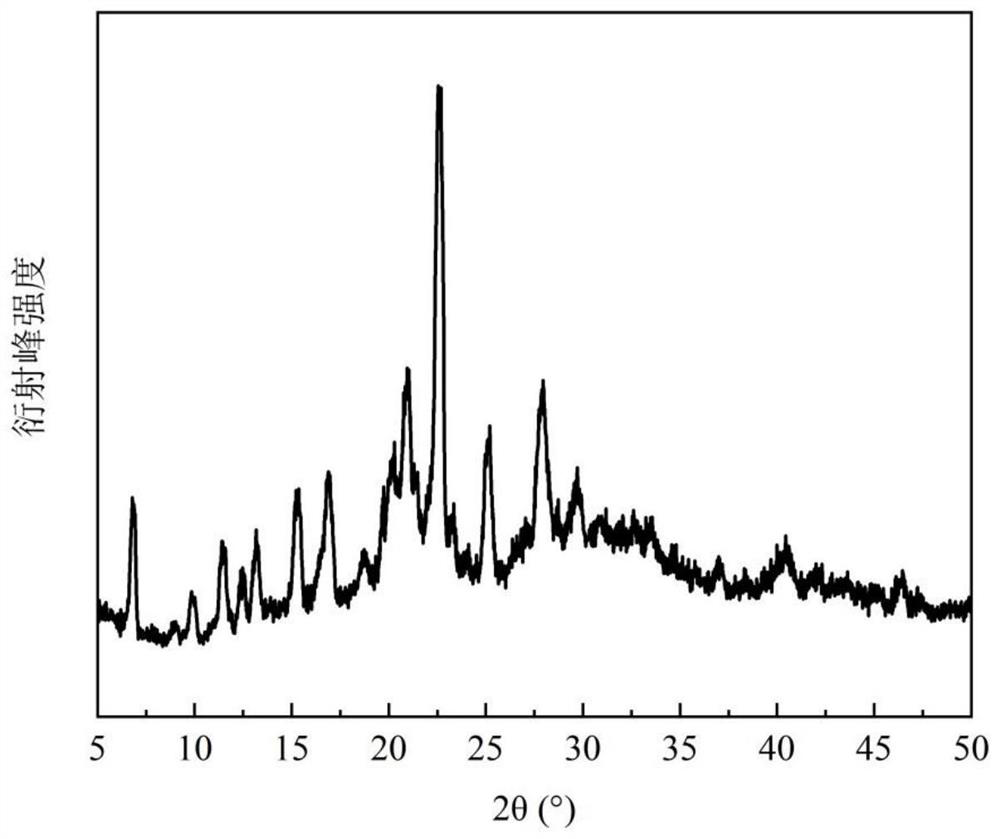
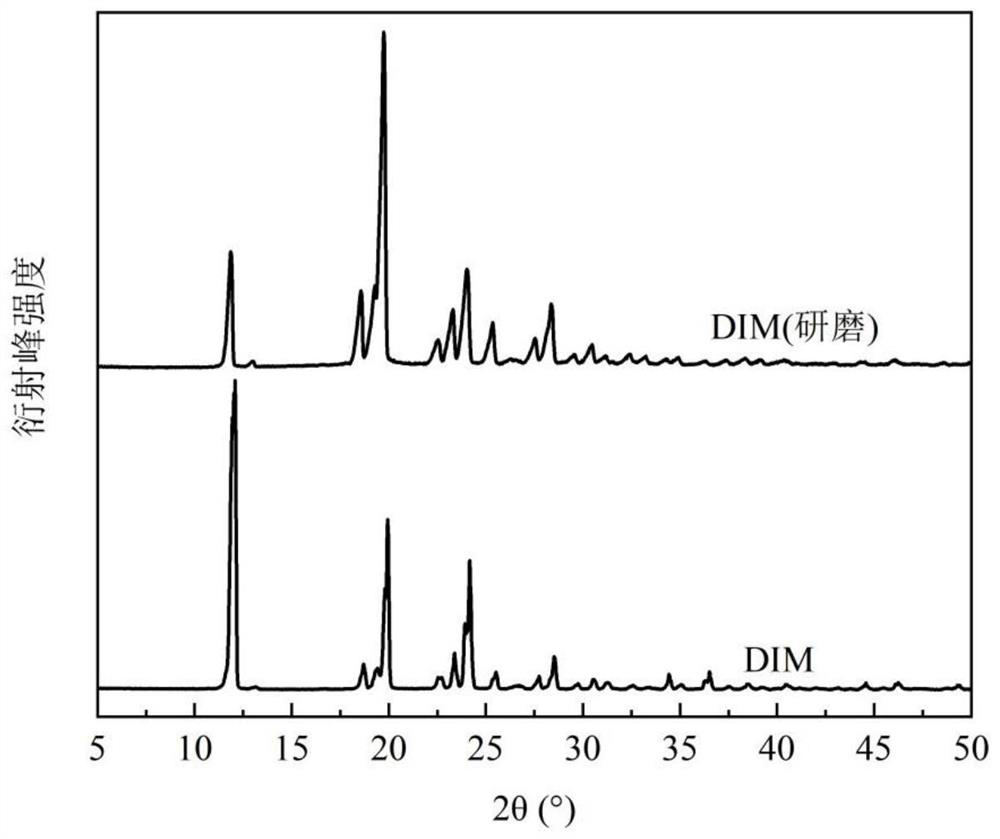
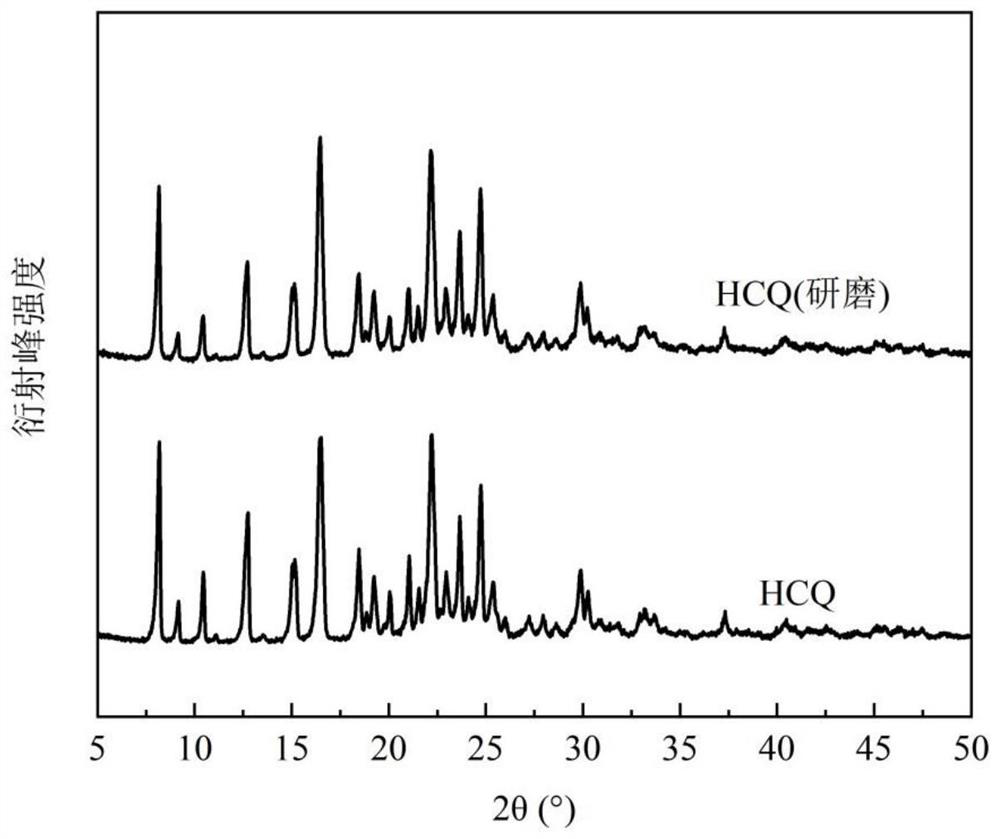
[0098]Dissolve 134.1mg of hydroxychloroquine (HCQ) and 220.8mg of 4-dimethylaminopyridine (DIM) in 2.0mL of acetonitrile at 25.1°C±0.1°C, stir to form a suspension, beat for 24h, let stand for 12h, and filter The solid was vacuum dried overnight at room temperature to obtain the new co-crystal of hydroxychloroquine. The X-ray powder diffraction pattern of the product shows that there are characteristic peaks at 6.80°, 11.45°, and 16.88° at the diffraction angle 2θ, and it does not have the characteristic peak of 8.12° hydroxychloroquine, nor does it have the characteristic of 4-dimethylaminopyridine at 12.09° peak, with attached figure 1 Consistent, confirm that the obtained product is a new co-crystal of hydroxychloroquine.
Embodiment 2
[0100] This embodiment has prepared a kind of new eutectic of hydroxychloroquine, and specific process is:
[0101] Dissolve 149.8mg of hydroxychloroquine and 267.8mg of 4-dimethylaminopyridine in 2.0mL of acetonitrile at 25.1°C±0.1°C, stir and form a suspension, beating for 24h, then standing for 12h, filtering, and vacuum-drying the solid at room temperature overnight to obtain The new co-crystal of hydroxychloroquine. The X-ray powder diffraction pattern of the product shows that there are characteristic peaks at 6.96°, 11.53°, and 17.07° at the diffraction angle 2θ, and it does not have the characteristic peak of 8.12° hydroxychloroquine, nor does it have the characteristic of 4-dimethylaminopyridine at 12.09° peak, with attached figure 1 Similarly, it was confirmed that the obtained product was a new cocrystal of hydroxychloroquine.
Embodiment 3
[0103] This embodiment has prepared a kind of new eutectic of hydroxychloroquine, and specific process is:
[0104] Dissolve 180.6 mg of hydroxychloroquine and 172.5 mg of 4-dimethylaminopyridine in 1.2 mL of acetonitrile at 34.2 °C ± 0.1 °C, stir to form a suspension, beat for 24 hours, and then let stand for 12 hours. After filtration, the solid is vacuum-dried at room temperature overnight to obtain The new co-crystal of hydroxychloroquine. The X-ray powder diffraction pattern of product shows that there are characteristic peaks at 6.80 °, 11.44 °, 17.02 ° ° with diffraction angle 2θ, and attached figure 1 Similarly, neither the characteristic peak of 8.12° hydroxychloroquine nor the characteristic peak of 4-dimethylaminopyridine 12.09° confirms that the resulting product is a new cocrystal of hydroxychloroquine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com