Interleukin-2 derivative as well as preparation method and application thereof
A technology of derivatives and fatty acid derivatives, applied in the field of interleukin-2 derivatives, can solve the problems of unstable product quality, poor patient compliance, prolonging IL-2 molecules, etc., achieve good drug prospects and reduce binding capacity , the effect of prolonging the half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: Preparation of IL-2K35C protein
[0050] According to the IL-2 wild-type sequence (SEQ ID NO: 1), the 35th lysine was mutated into cysteine, and the mutated protein was named IL-2K35C (SEQ ID NO: 2); in addition, the The 43rd lysine and the 64th lysine were mutated into cysteine respectively, and named as IL-2K43C and IL-2K64C for comparison. In order to facilitate subsequent purification, the Fc region of human IgG1 was introduced at the N-terminal of the protein, and the thrombin cleavage site LVPRGS was introduced between Fc and the target protein. The full-length sequence of IL-2K35 with Fc tag (Fc-IL-2K35C) is shown in SEQ ID NO:3.
[0051] The above clone design was entrusted to Taizhou Baiying Biotechnology Co., Ltd. to construct and perform CHO cell expression and purification. Using a 125ml disposable sterile shaker flask, thaw the frozen CHO cells in a 37-degree water bath, and dilute the cells to 0.3×10 6 pcs / ml, volume 30ml. 125rpm (19mm ...
Embodiment 2
[0053] Embodiment 2: the synthesis of fatty acid derivative
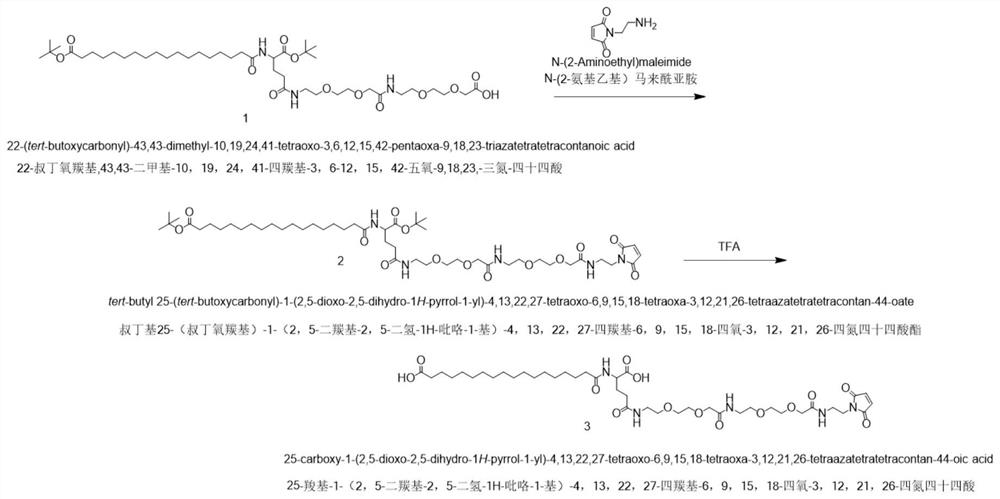
[0054] Such as image 3 Shown, with 22-(tert-butoxycarbonyl)-43,43-dimethyl-10,19,24,41-tetracarbonyl-3,6,12,15,42-pentaoxo-9,18,23 Triazine-tetradecanoic acid (1) is a raw material (hereinafter referred to as semaglutide side chain intermediate, synthesized with reference to Example 1 of patent document WO2011117415A) and N-(2-aminoethyl) maleimide in The condensing agent 2-bromo-1-ethylpyridine tetrafluoroborate (Bide Pharmaceuticals, BKZ779) reacted to obtain tert-butyl 25-(tert-butoxycarbonyl)-1-(2,5-carbonyl-2, 5-dihydro-1H-pyrrol-1-yl)-4,13,22,27-tetracarbonyl-6,9,15,18-tetraoxo-3,12,21,26-tetraazotetratradecanoic acid Ester (2), followed by removal of Boc protection with trifluoroacetic acid (TEDIA, TS4295-013) to give the fatty acid derivative 25-carboxy-1-(2,5-dicarbonyl-2,5-dihydro-1H-pyrrole -1-yl)-4,13,22,27-tetracarbonyl-6,9,15,18-tetraoxo-3,12,21,26-tetraazatetradecanoic acid (3). The specific oper...
Embodiment 3
[0055] Example 3: Conjugation of fatty acid derivatives and IL-2K35C
[0056] TCEP (Suzhou Haofan Biological Co., Ltd., 20190501, prepared as an aqueous solution with reducing buffer) and IL-2K35C protein were mixed at a molar ratio of 6:1, and reduced at 18°C for two hours to make the mutated surface half The sulfhydryl group of cystine is in a free state. The reducing buffer was 35mM sodium citrate (Sangon, A610035), 2mM EDTA (Sangon, A610185-0500), 154mM NaCl (Sangon, A501218-0001), pH=5. After the reduction was completed, the product was ultracentrifuged 10 times at a speed of 7000 rpm / min, and 10 times the buffer volume was replaced to remove TCEP. The fatty acid derivative (FA) obtained in Example 2 was dissolved in a reducing buffer containing 5% N,N-dimethylformamide (DMA) (TEDIA, DS1441-001), and the reduced IL-2K35C The mixture was mixed at a molar ratio of 2:1, and the coupling reaction was carried out at 18° C., and the reaction time was 2 hours.
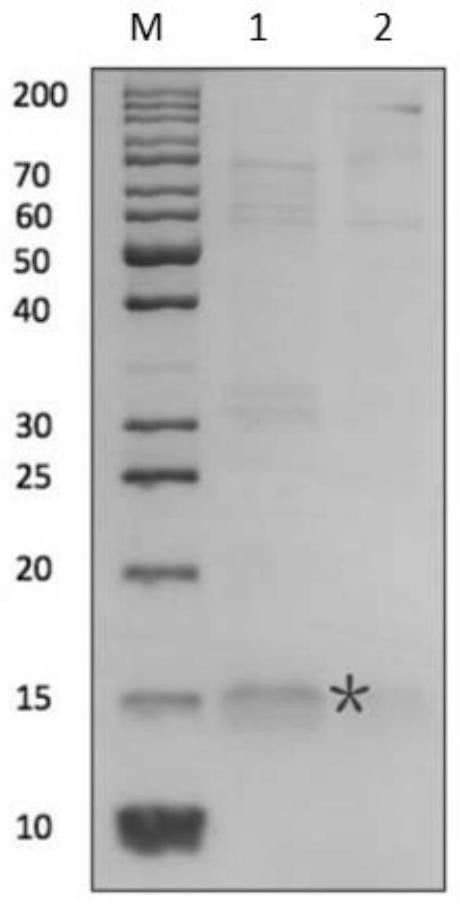
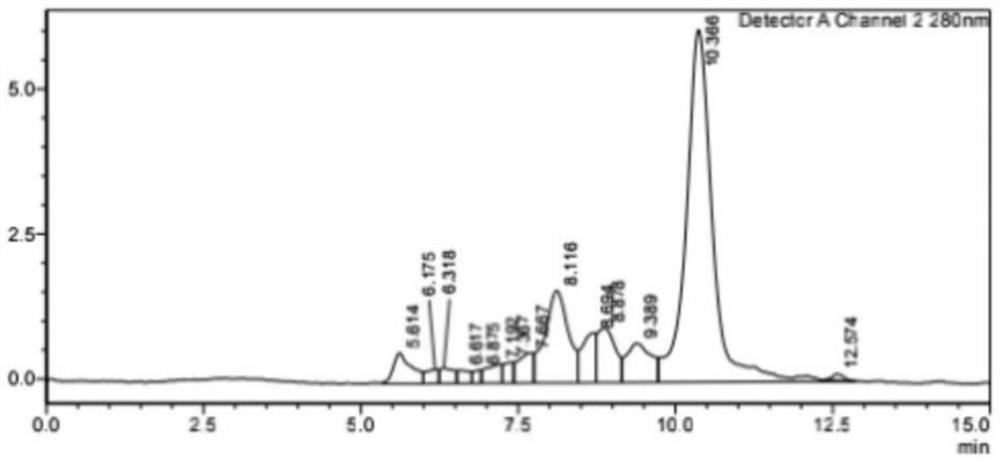
[0057] Afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com