Kit and method for detecting concentration of cyclosporine drug in dried blood spot sample
A technology for cyclosporine and drug concentration, which is applied in the direction of measuring devices, types of packaging items, special packaging items, etc., can solve the problems of interference of test results and increased volume, and achieve convenient operation and portability, avoid cross-contamination, and eliminate influence Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
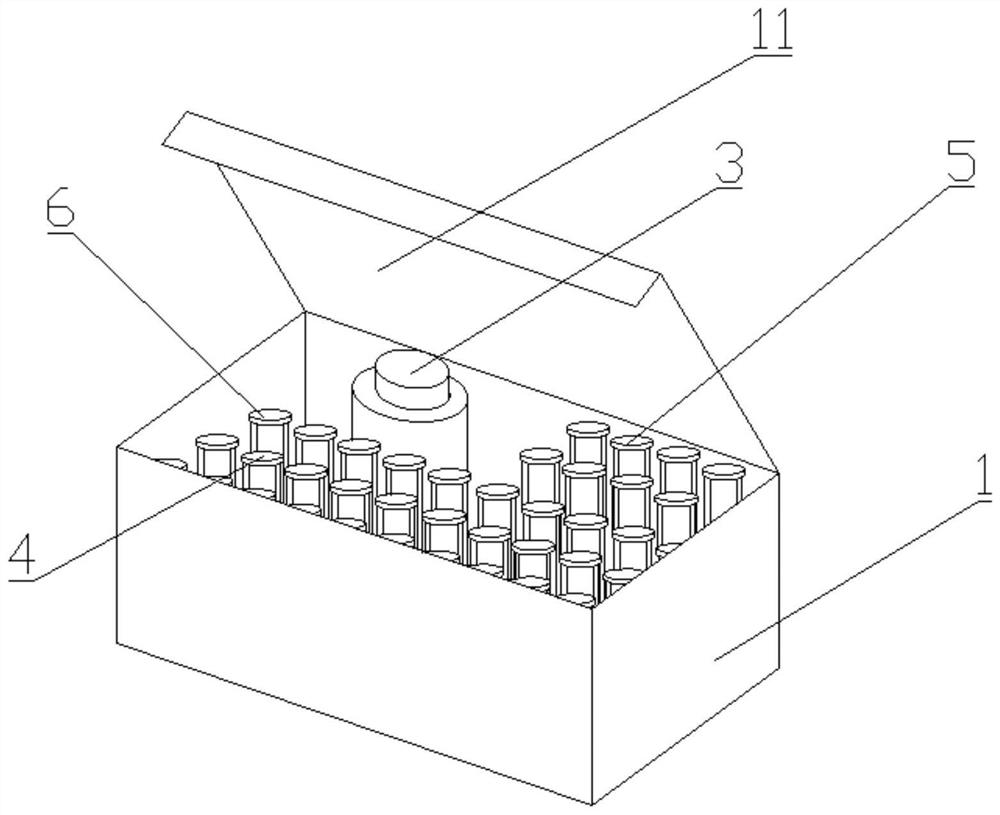
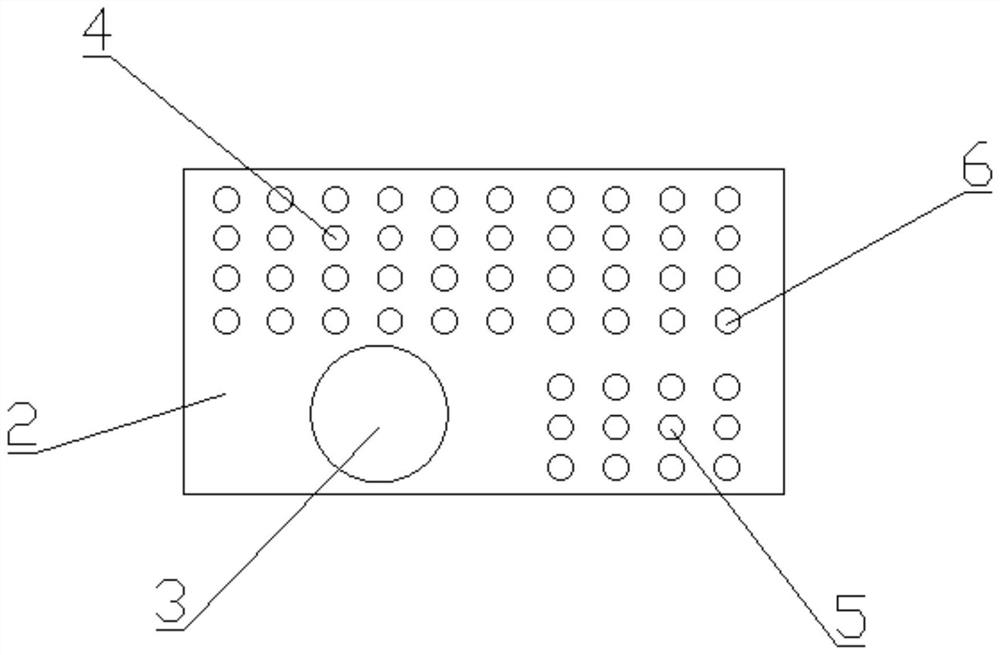
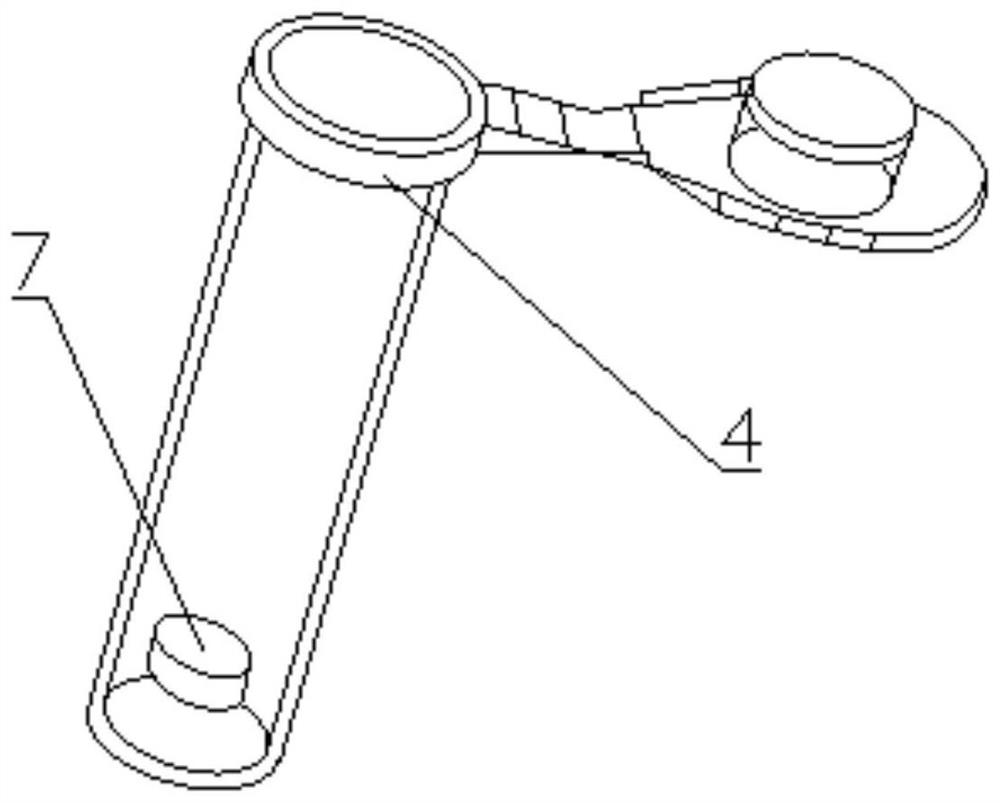
[0043] Such as Figure 1-5 As shown, a kit for detecting the drug concentration of cyclosporine in dried blood film samples, including box body 1, inner liner 2, sample extract, dried blood film cyclosporine calibrator, dried blood film cyclosporine The quality control product and the blank quality control product are characterized in that the inner liner 2 is arranged at the bottom end inside the box body 1, and the inner liner 2 is provided with a plurality of accommodating grooves, and jars 3, The first sample collection tube 4, the second sample collection tube 5 and the third sample collection tube 6, the sample extract is stored in the inside of the jar 3, and the dried blood spot cyclosporine calibrator is stored in the first sample collection tube. Inside the tube 4, the dry blood spot cyclosporine quality control product is stored in the second sample collection tube 5, the blank quality control product is stored in the third sample collection tube 6, the first sample...
Embodiment 2
[0053] The composition and preparation method of embodiment two kits
[0054] Based on a test kit in Example 1, the specific components and specific concentrations of the test kit in this embodiment are shown in the following table:
[0055]
[0056] This kit is attached with high, medium and low concentrations of dry blood film cyclosporine quality control substances for quality control of the test results, and a blank control for the specificity and specificity evaluation of cyclosporine , Monitoring of high-concentration sample residues.
[0057] The specific preparation method of the kit is as follows:
[0058] (1) Assembly of sample collection tube and filter paper
[0059] Place the glass fiber filter paper 7 at the bottom of the sample collection tube with tweezers.
[0060] (2) Assembly of desiccant
[0061] Fold the drying sheet and place it in a fixed tube in a low-humidity workshop.
[0062] (3) Preparation of calibrators and quality control products
[006...
Embodiment 3
[0083] Example 3 Using this kit to detect the concentration of cyclosporine in dried blood samples
[0084] The kit for detecting the drug concentration of cyclosporine in the dried blood film sample is used as a carrier, and the internal standard method is used in combination with liquid chromatography tandem mass spectrometer to quantitatively detect the drug concentration of cyclosporine in the dried blood film sample, including the following steps:
[0085] 1) Sample pretreatment:
[0086] First take two third sample collection tubes, add 300 μL of acetonitrile and water mixed solution to one as a double blank sample, the volume ratio of acetonitrile and water is 7:3, and add 300 μL of sample extract to the other as a single blank sample ;
[0087] Then take the first sample collection tube, the second sample collection tube and the sample collection tube of the dried blood spot sample to be tested, add 300 μL of sample extract, let all the sample collection tubes stand f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com